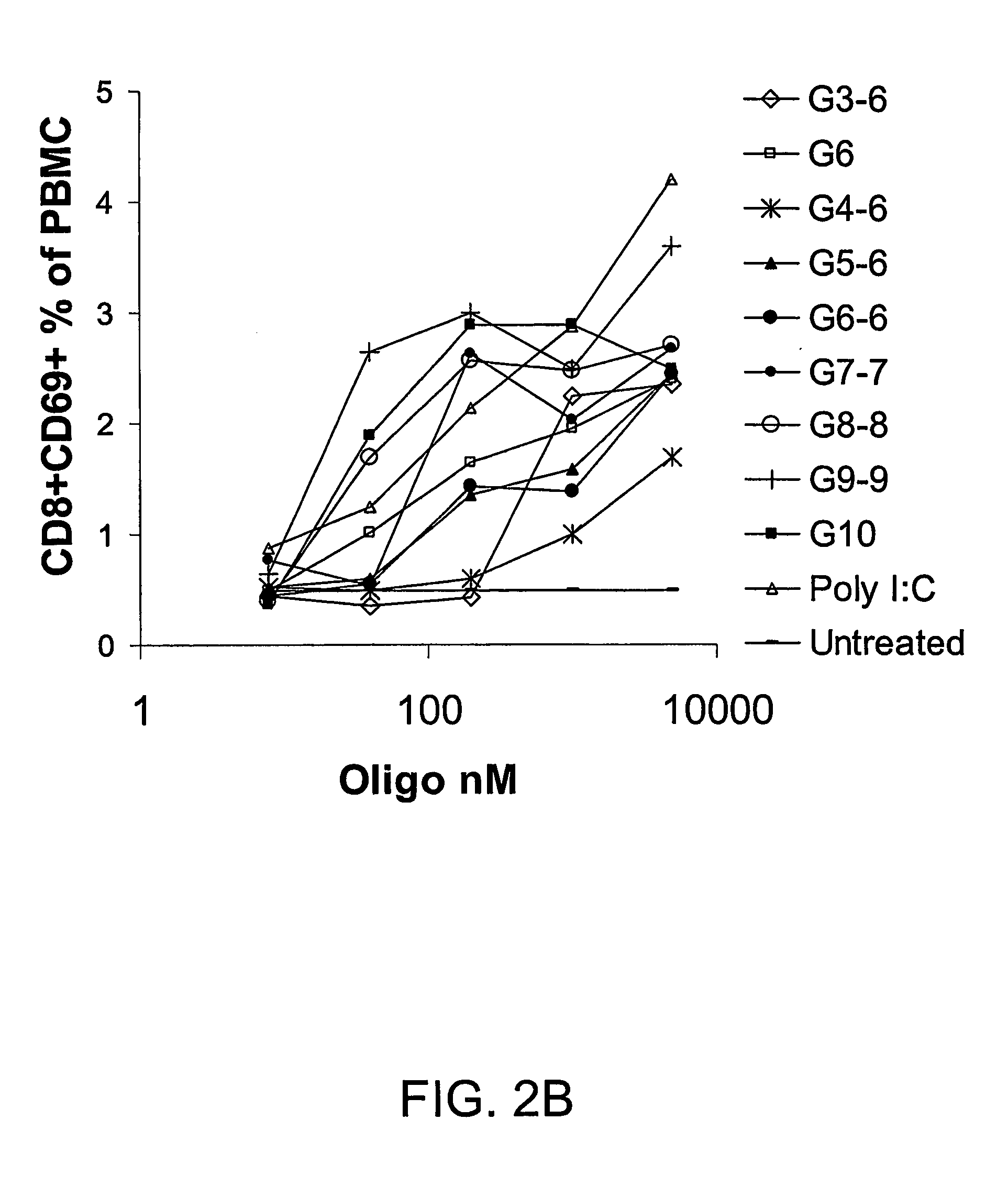
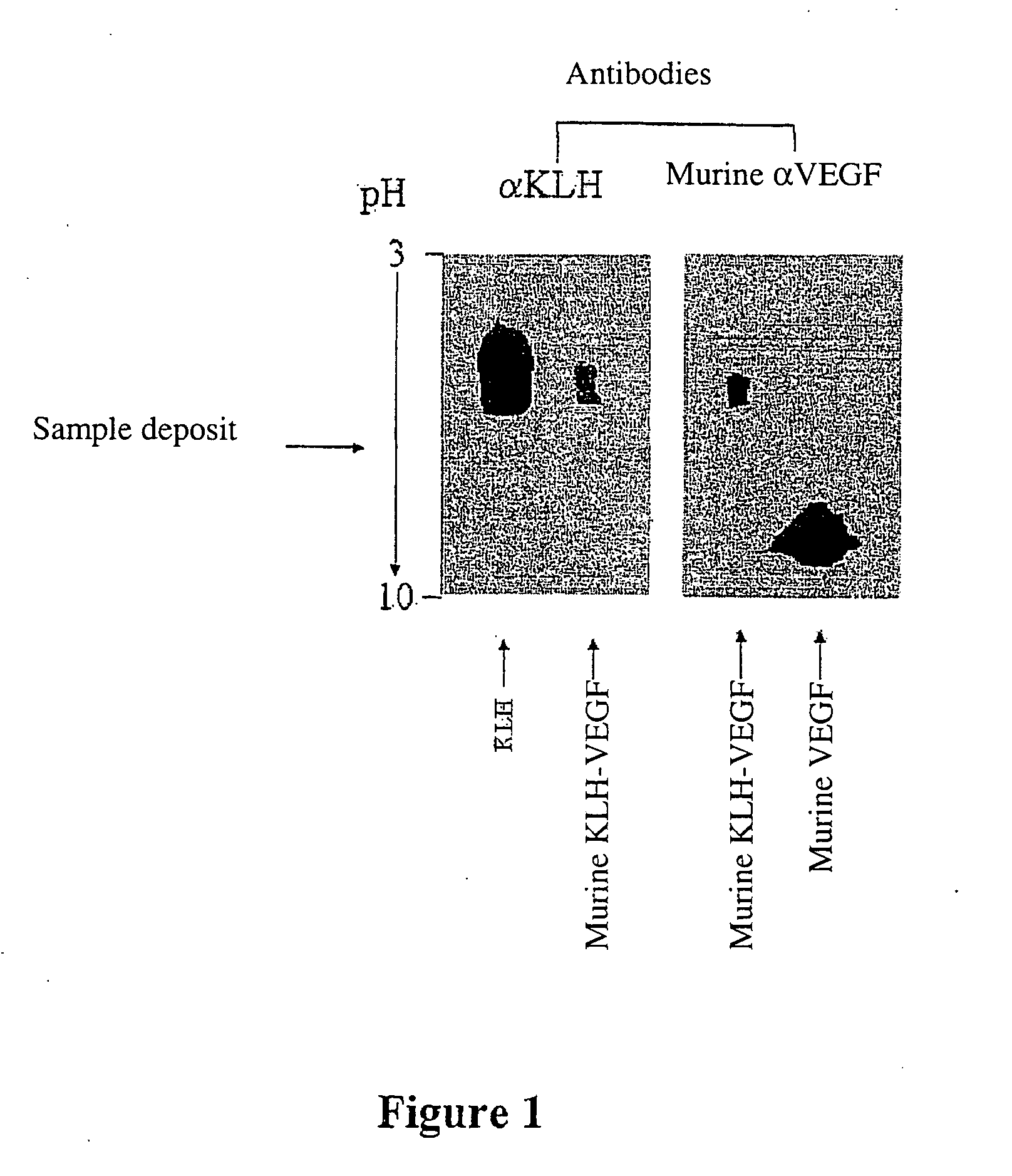
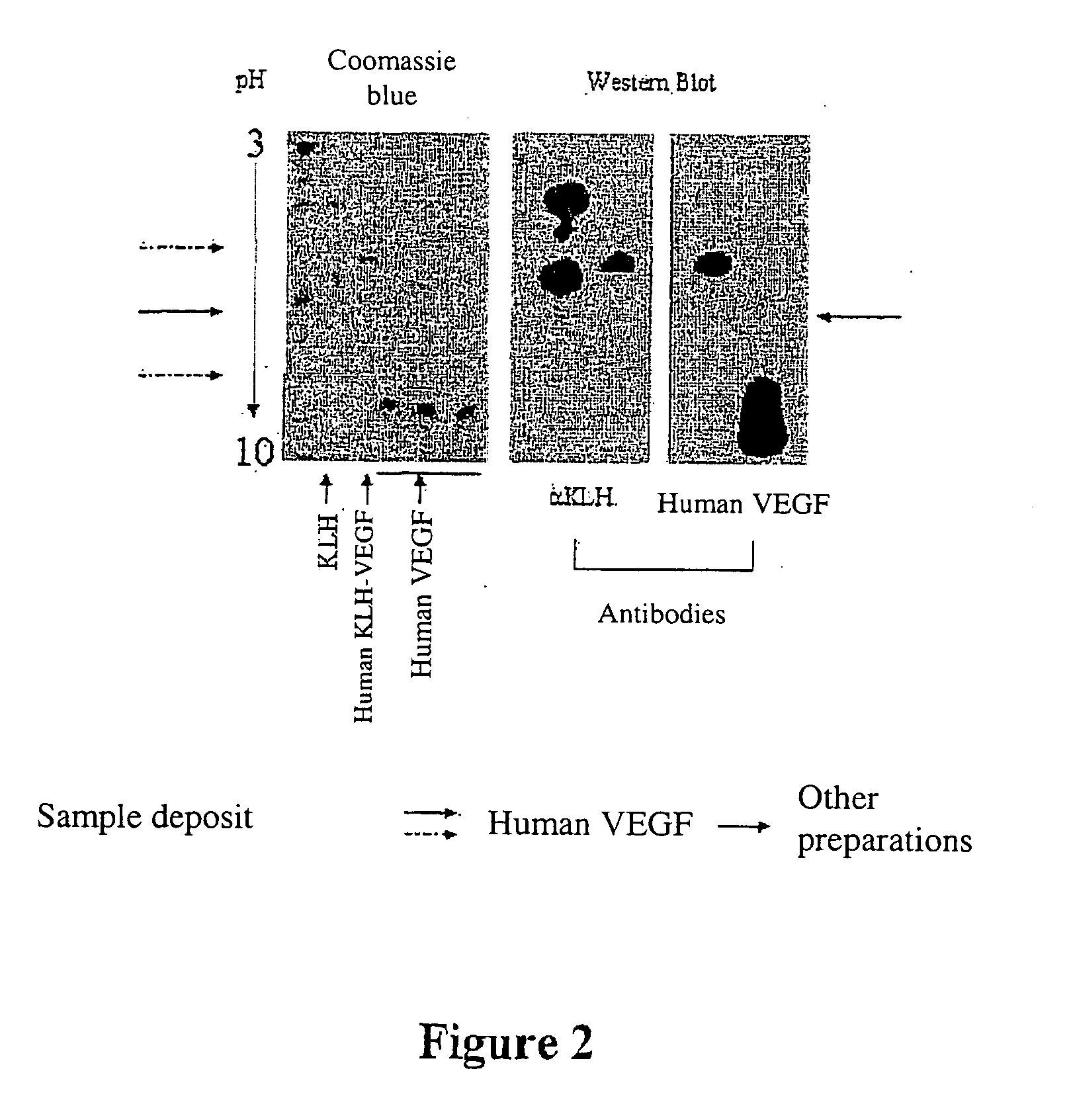
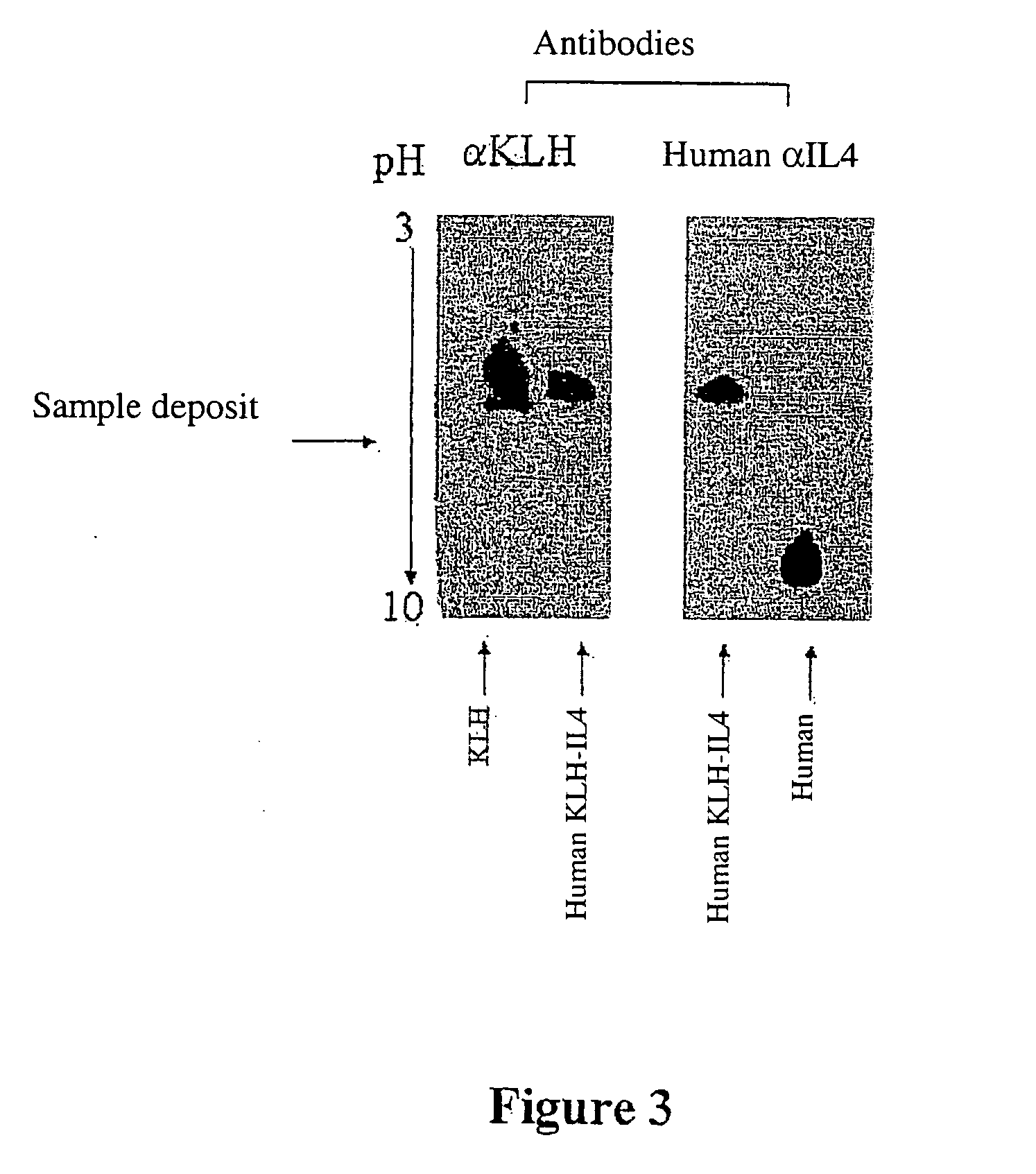
Patents
Literature
128results about "Antigen-carrier link" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
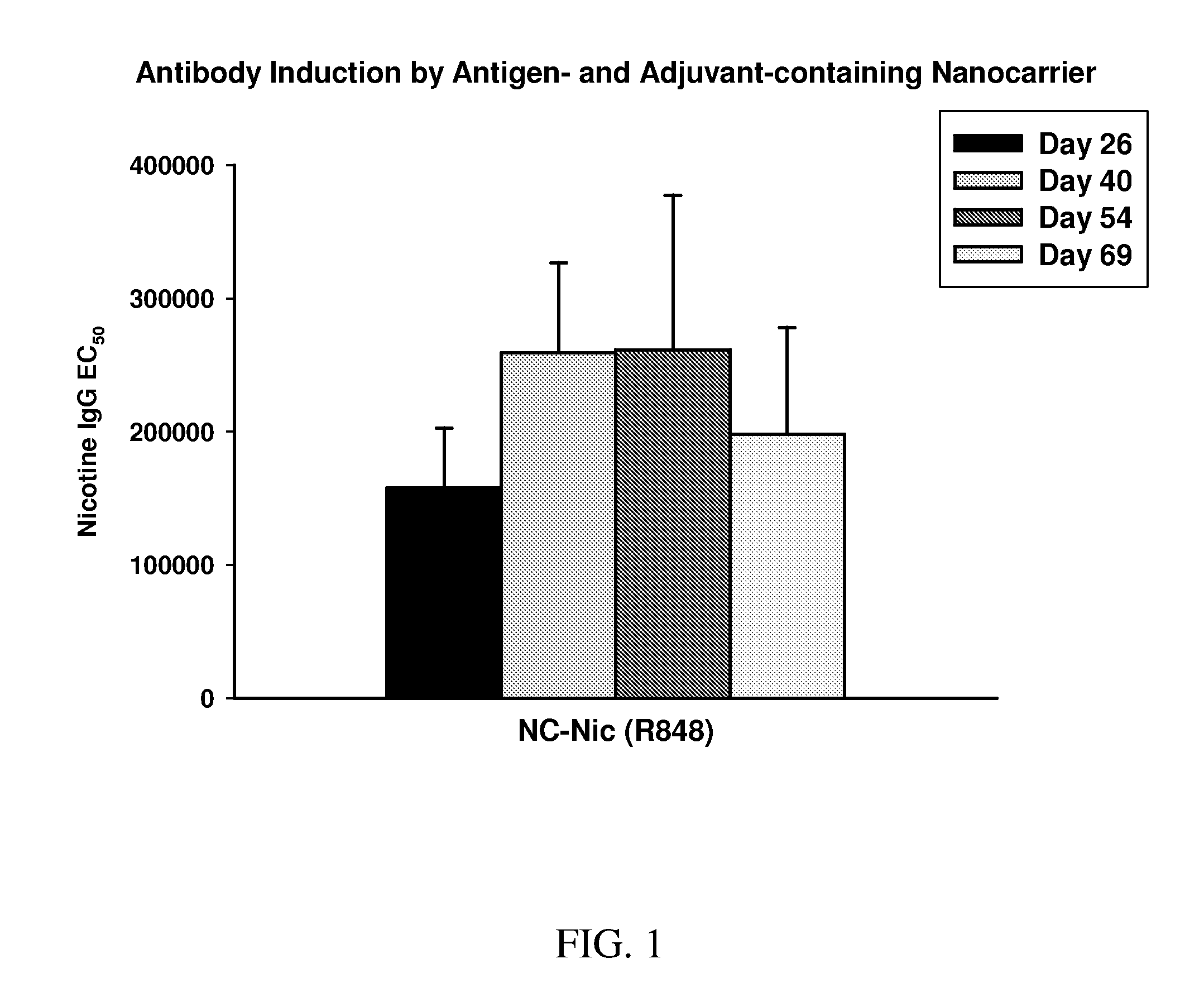
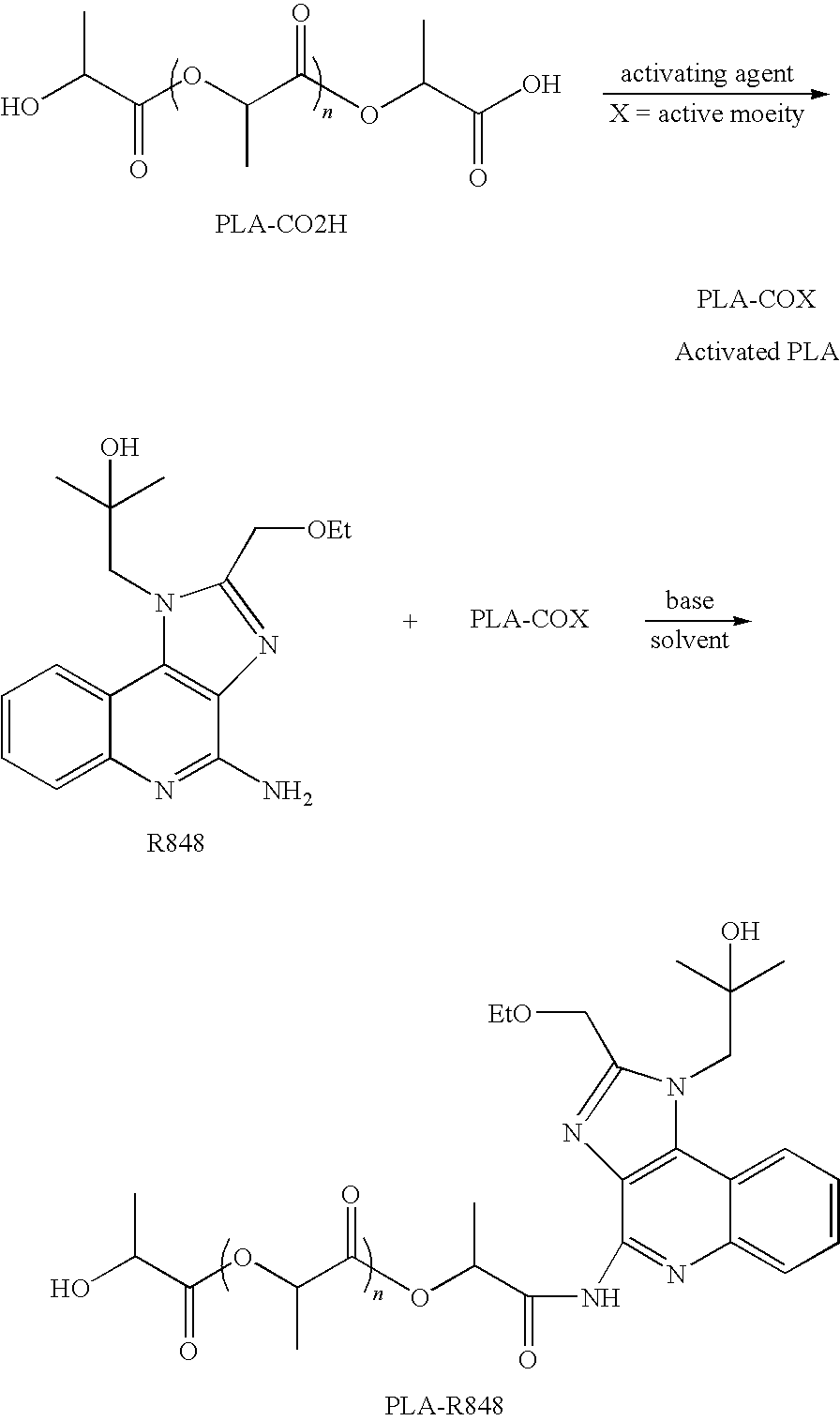
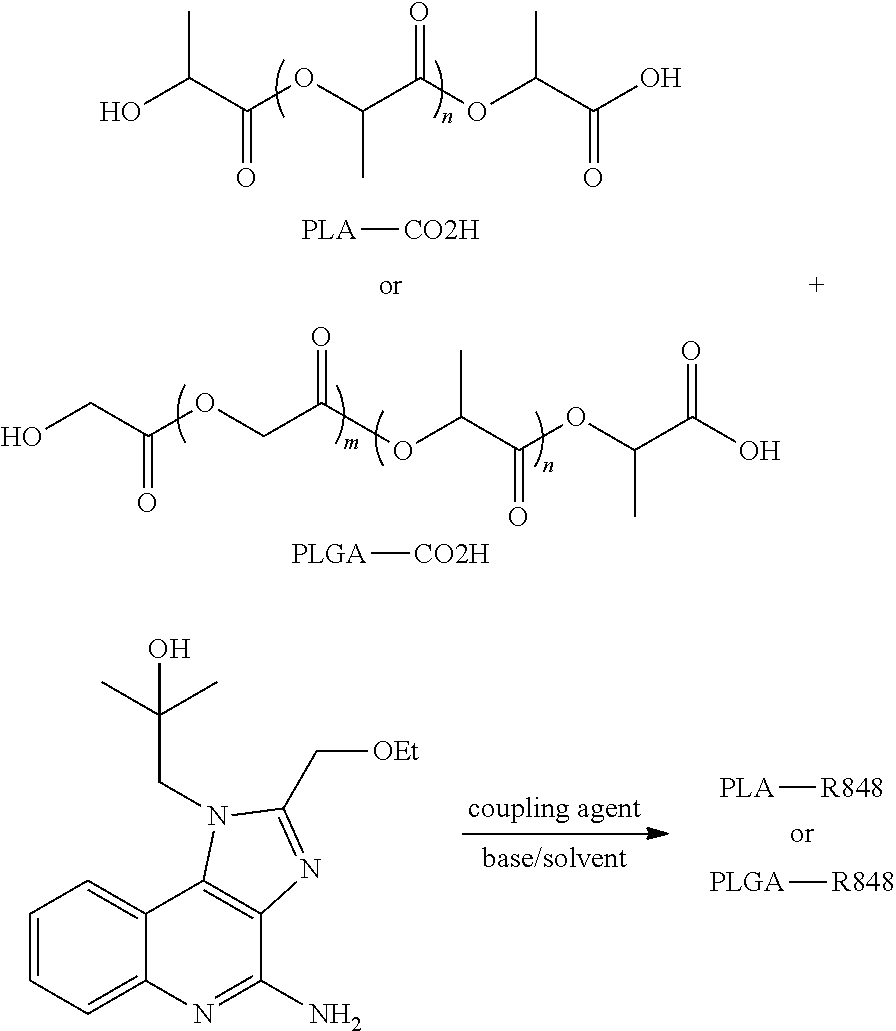
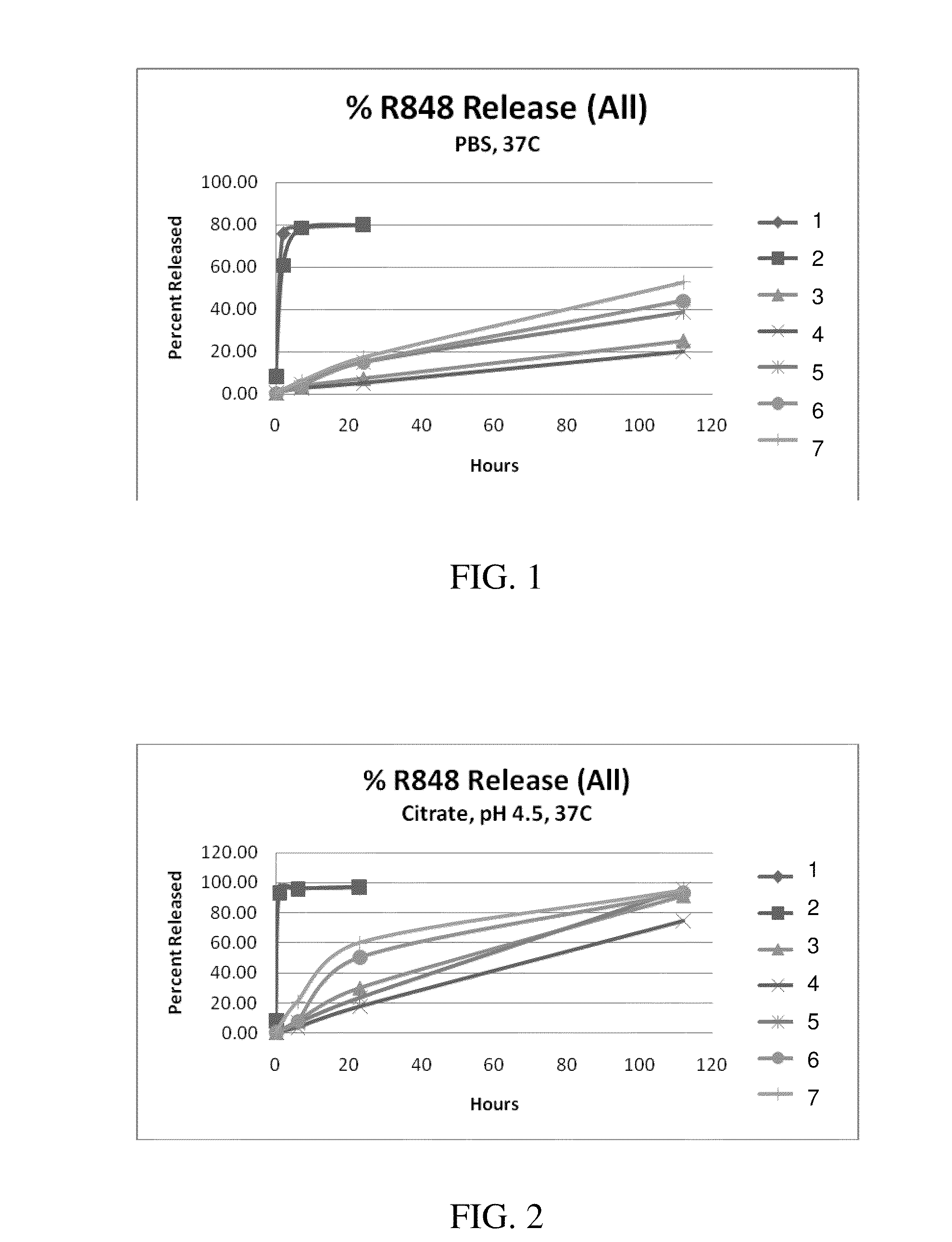
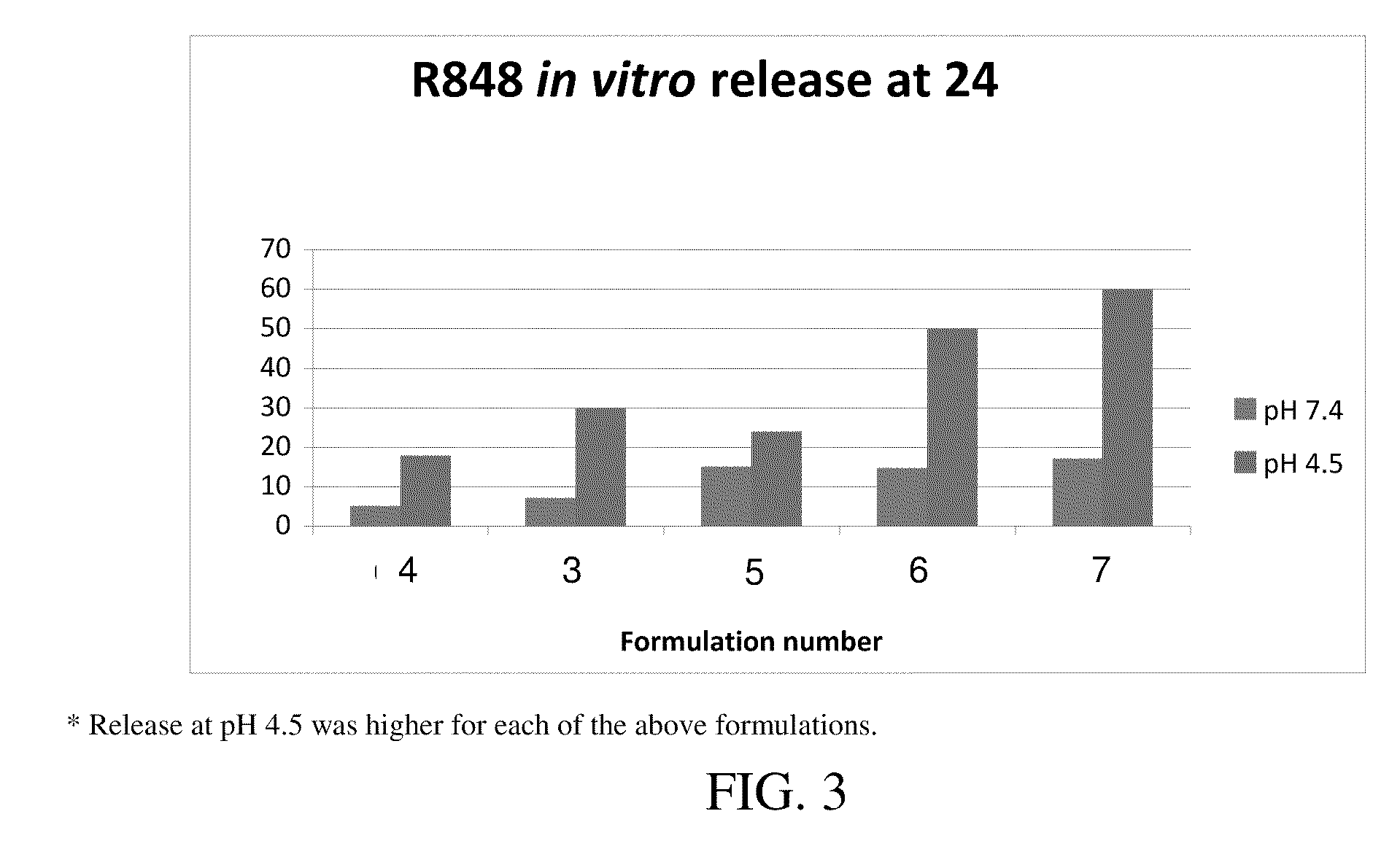
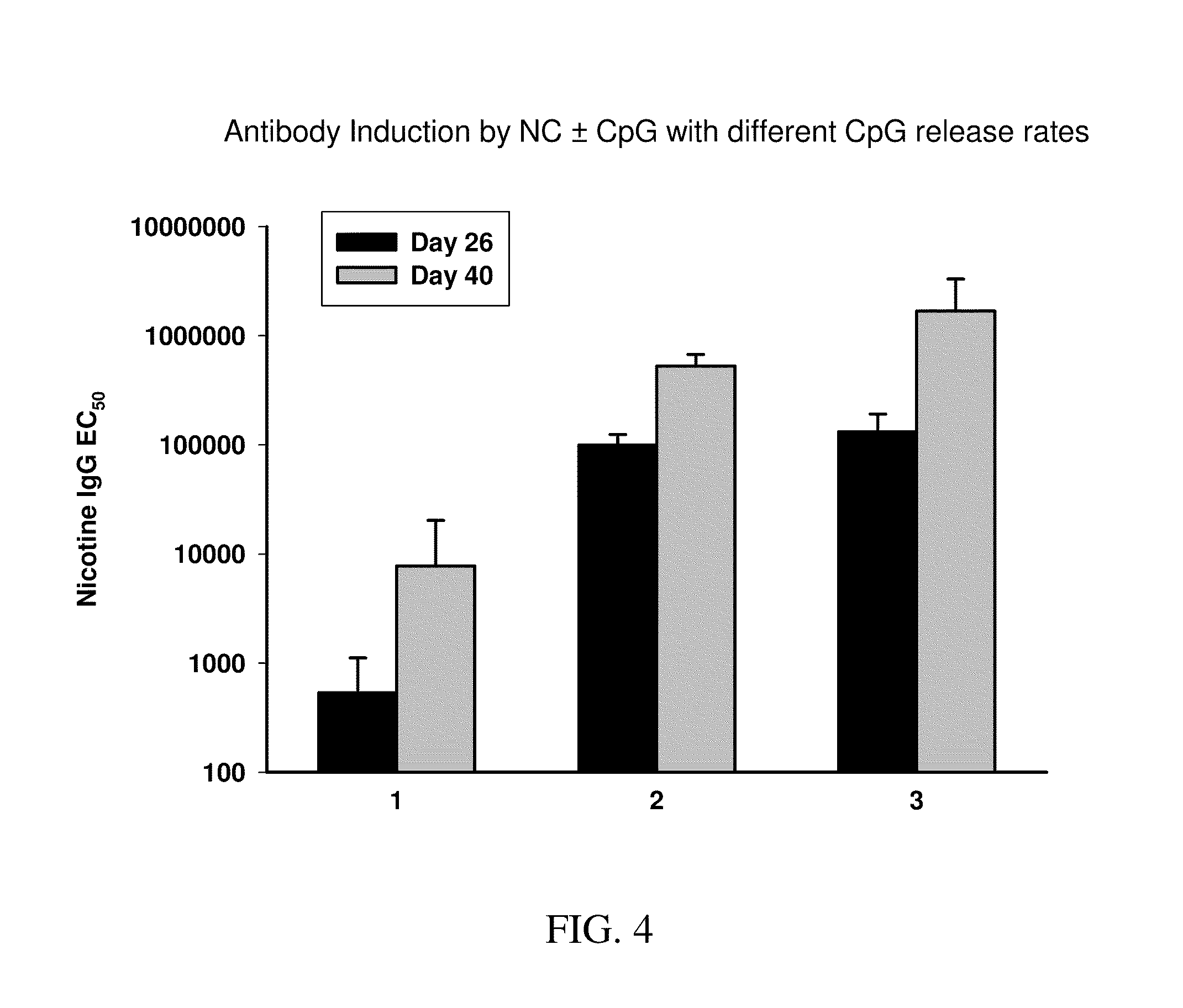
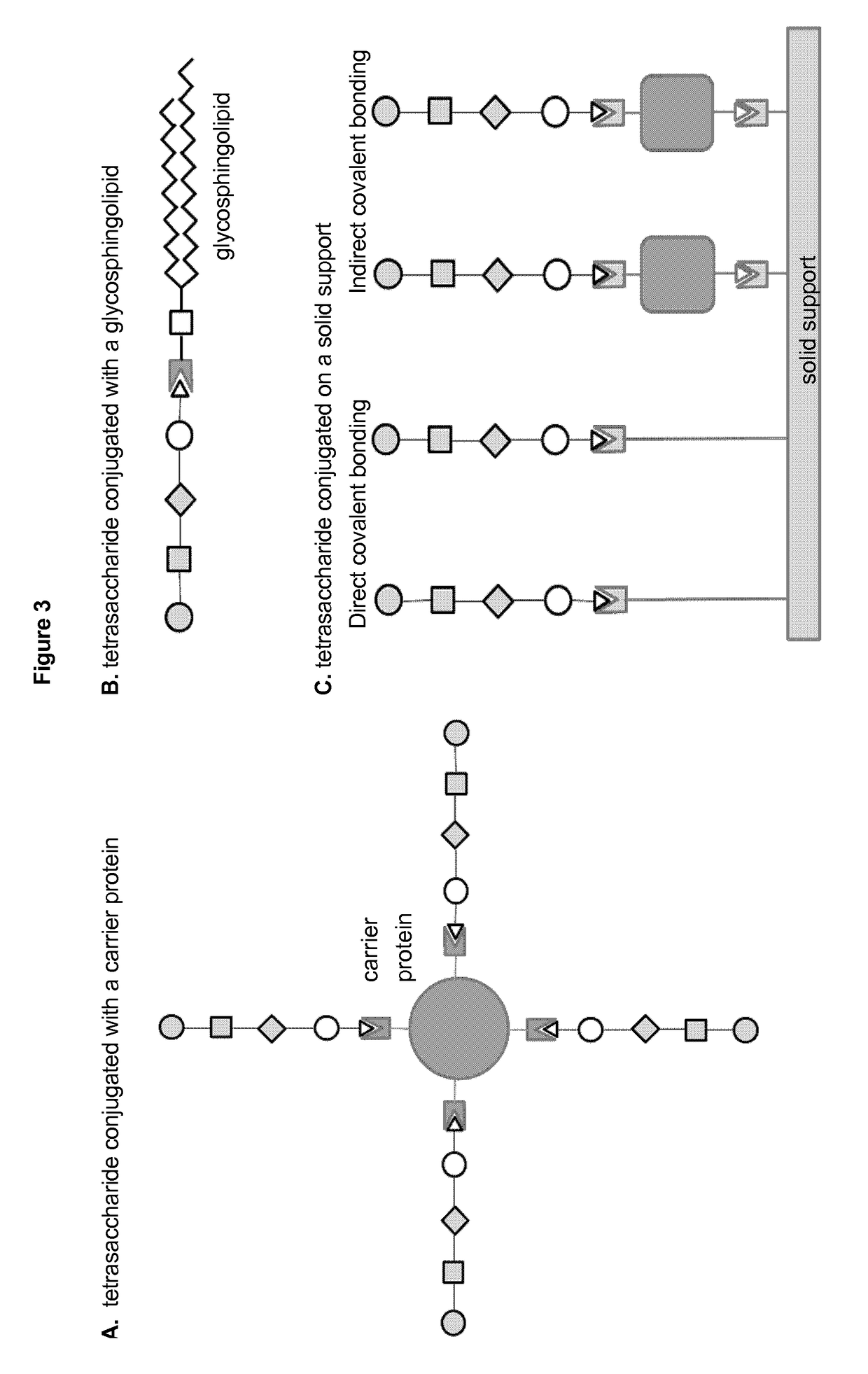
Nanocarriers possessing components with different rates of release
InactiveUS20100303850A1Induce and enhance immune responseStrong and long-term humoral immune responsePowder deliveryNervous disorderAntigenNanocarriers
This invention relates to compositions, and related methods, of synthetic nanocarriers that comprise immunomodulatory agents and antigens that are differentially released from the synthetic nanocarriers.
Owner:SELECTA BIOSCI
Targeted synthetic nanocarriers with ph sensitive release of immunomodulatory agents
This invention relates to compositions, and related methods, of synthetic nanocarriers that target sites of action in cells, such as antigen presenting cells (APCs), and comprise immunomodulatory agents that dissociate from the synthetic nanocarriers in a pH sensitive manner. Also disclosed are compositions and methods relating to synthetic nanocarriers that encapsulate labile immunomodulatory agents that dissociate from the synthetic nanocarriers in a pH sensitive manner.
Owner:SELECTA BIOSCI
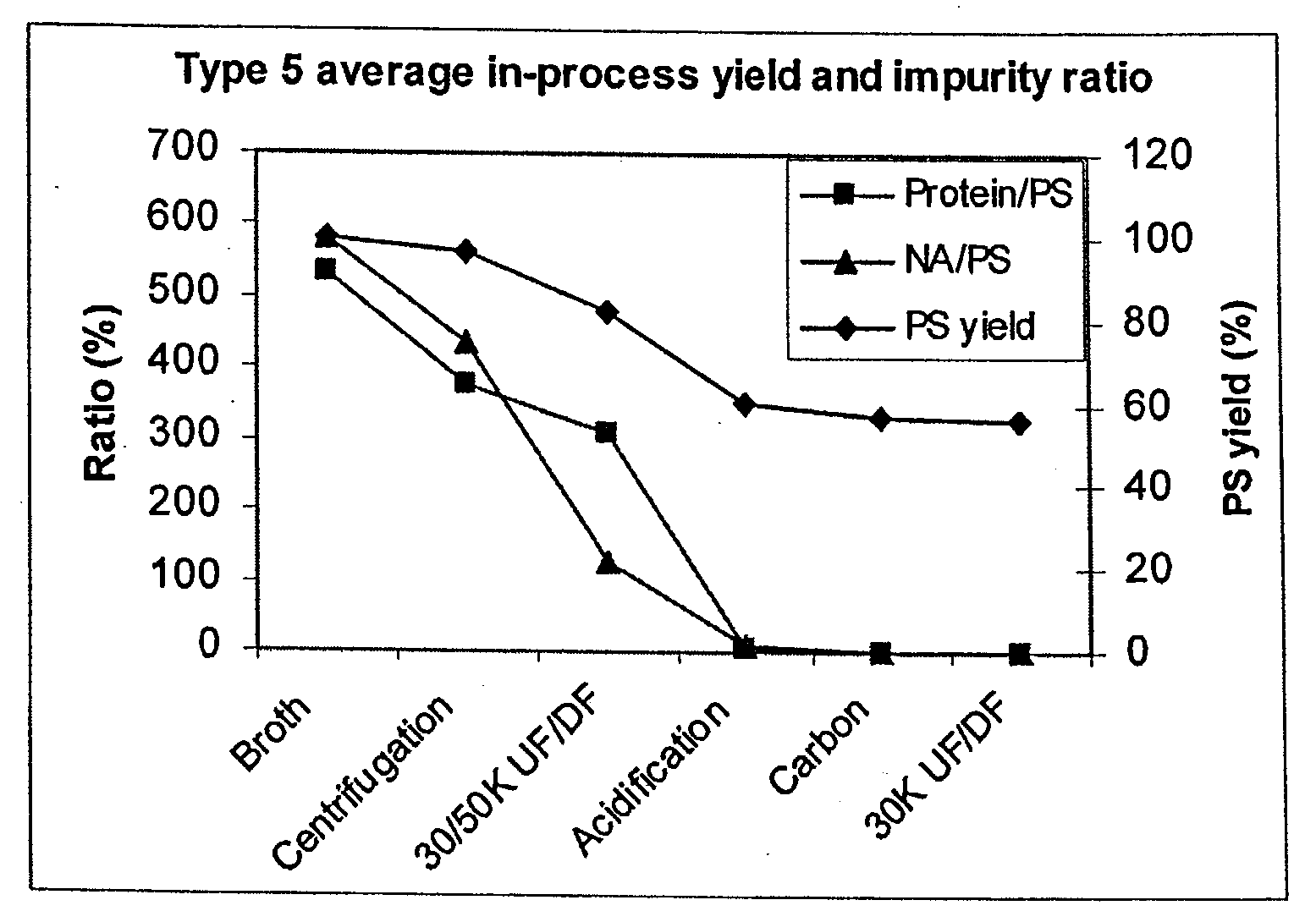
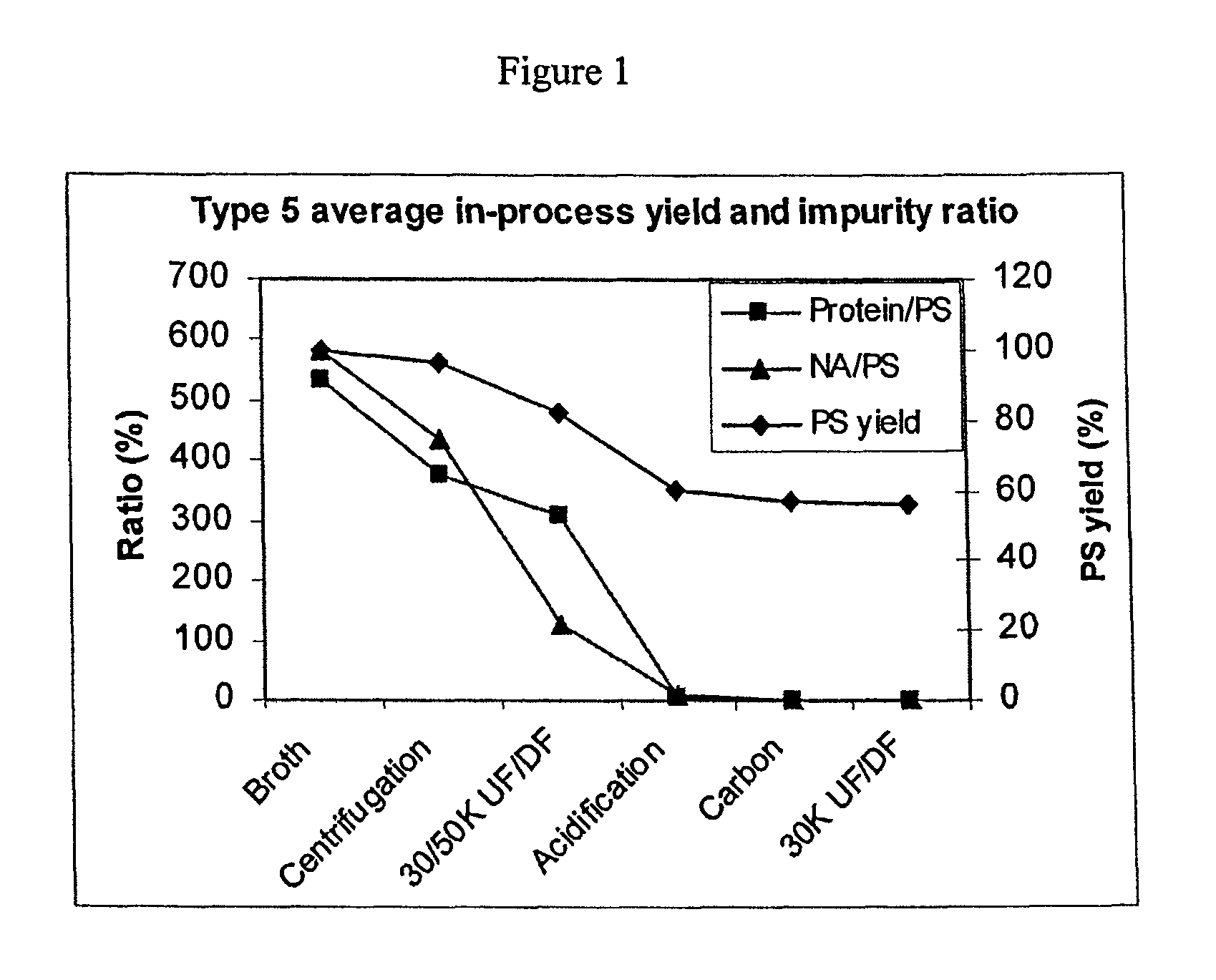
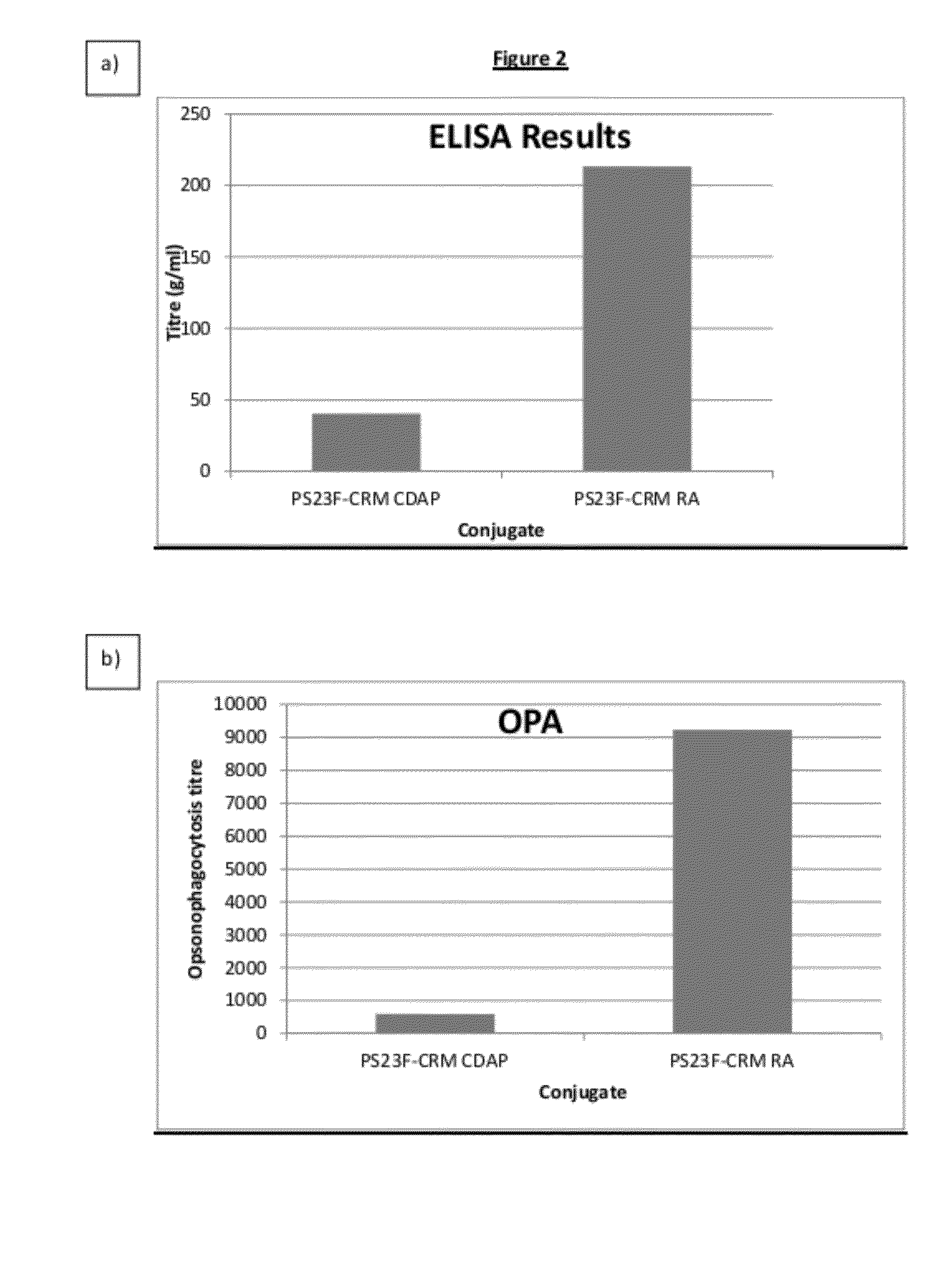
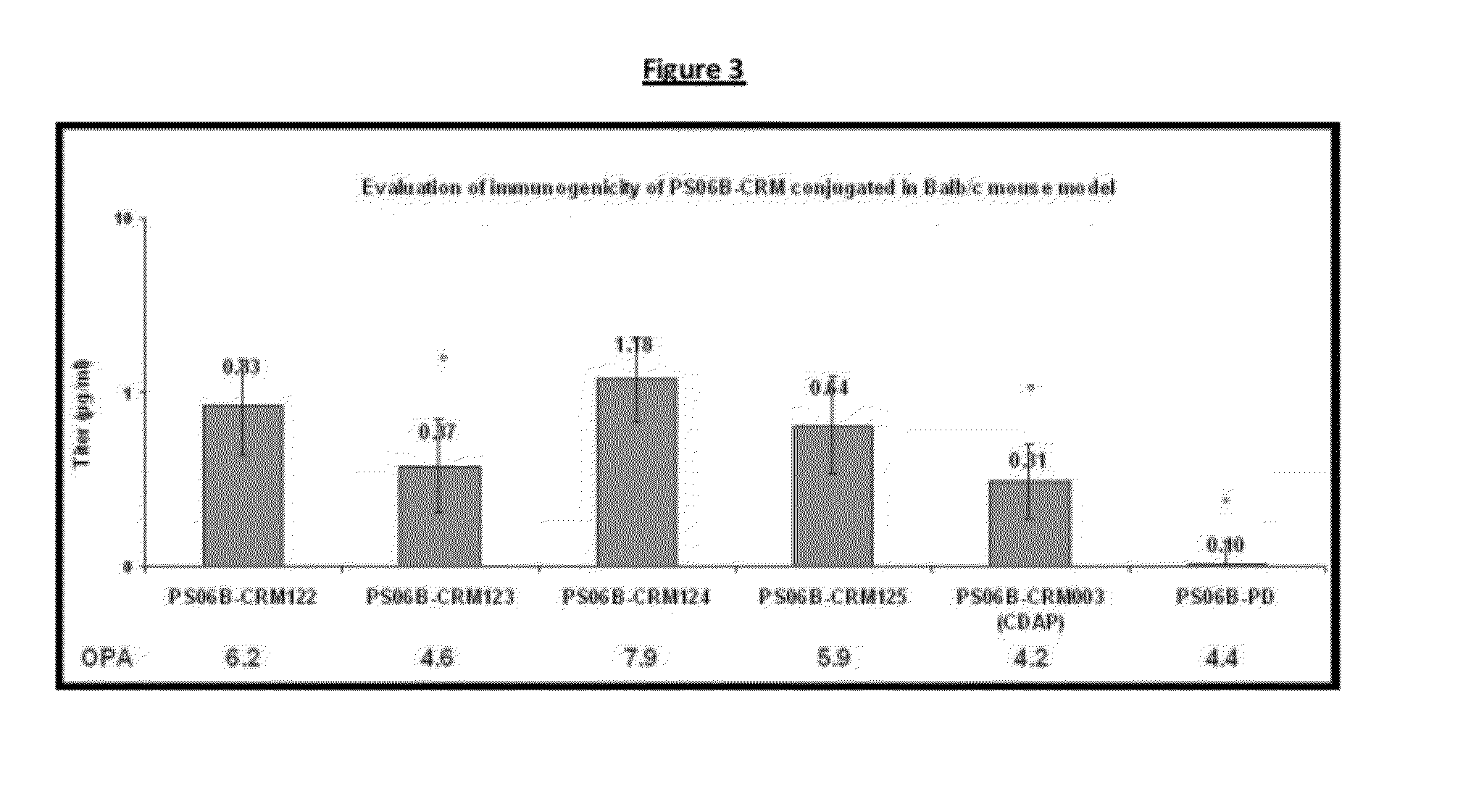
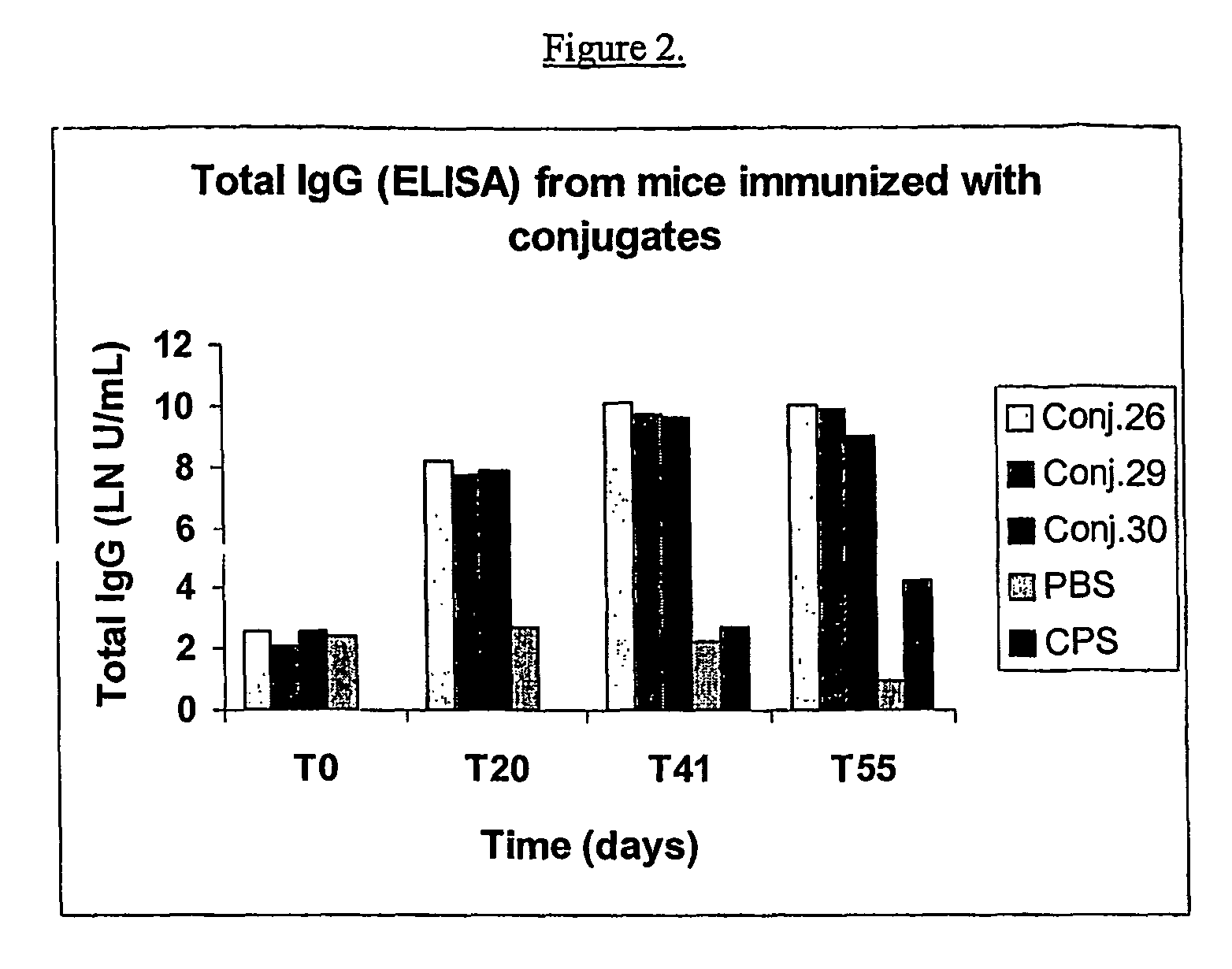
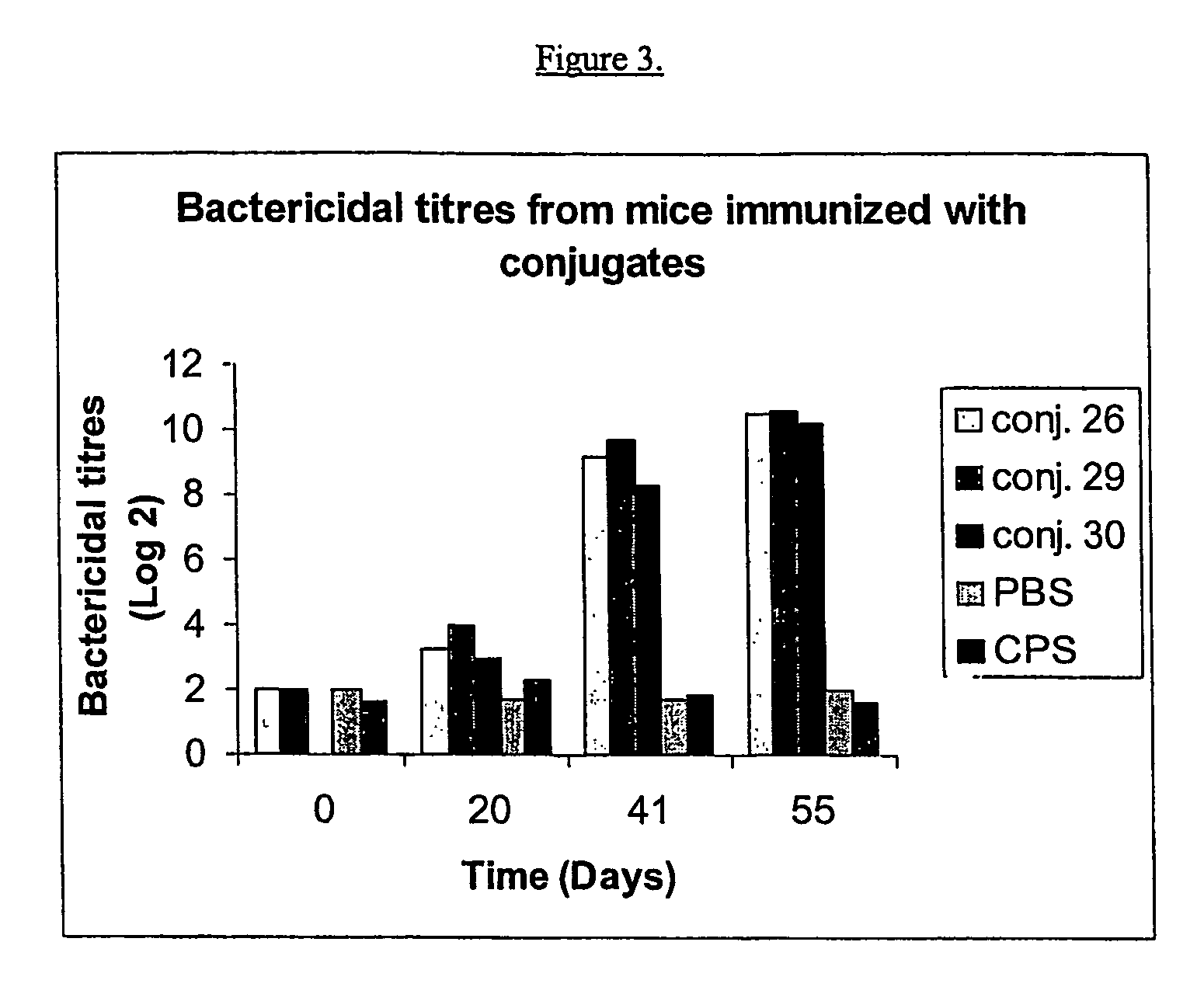
Shortened purification process for the production of capsular streptococcus pneumoniae polysaccharides
ActiveUS20080286838A1Increase polysaccharide concentrationReduce the presence of impuritiesAntibacterial agentsSugar derivativesPurification methodsActivated carbon filtration
A shortened process for producing a solution containing substantially purified capsular polysaccharides from a cellular Streptococcus pneumoniae lysate broth is described. Ultrafiltering and diafiltering a clarified S. pneumoniae lysate followed by pH adjustment to less than 4.5, preferably about 3.5, precipitated at least 98% of the protein in the solution without seriously affecting polysaccharide yield. Furthermore, following ultrafiltration and diafiltration and acidification to a pH of less than 4.5, filtration using activated carbon precipitated at least 90% of remaining protein without seriously affecting polysaccharide yield. Exemplary, non-limiting S. pneumoniae serotypes that can be purified using the shortened process of the invention are 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. In one embodiment, the Streptococcus pneumoniae cells are lysed using deoxycholate sodium (DOC), while in another embodiment the lytic agent is a non-animal derived lytic agent such as N-lauryl sarcosine sodium (NLS).
Owner:WYETH LLC
A-beta immunogenic peptide carrier conjugates and methods of producing same
InactiveUS20080145373A1Nervous disorderNervous system antigen ingredientsImmunogenicityImmunogenic peptide
Owner:JANSSEN SCI IRELAND UC +1
Pneumococcal Polysaccharide Conjugate Vaccine
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid (DT) or CRM197, optionally wherein 19F is the only saccharide in the composition conjugated to diphtheria toxoid (DT) or CRM197.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Beta immunogenic peptide carrier conjugates and methods of producing same
InactiveUS20070161088A1Reserved functionNervous disorderPeptide/protein ingredientsImmunogenicityImmunogenic peptide
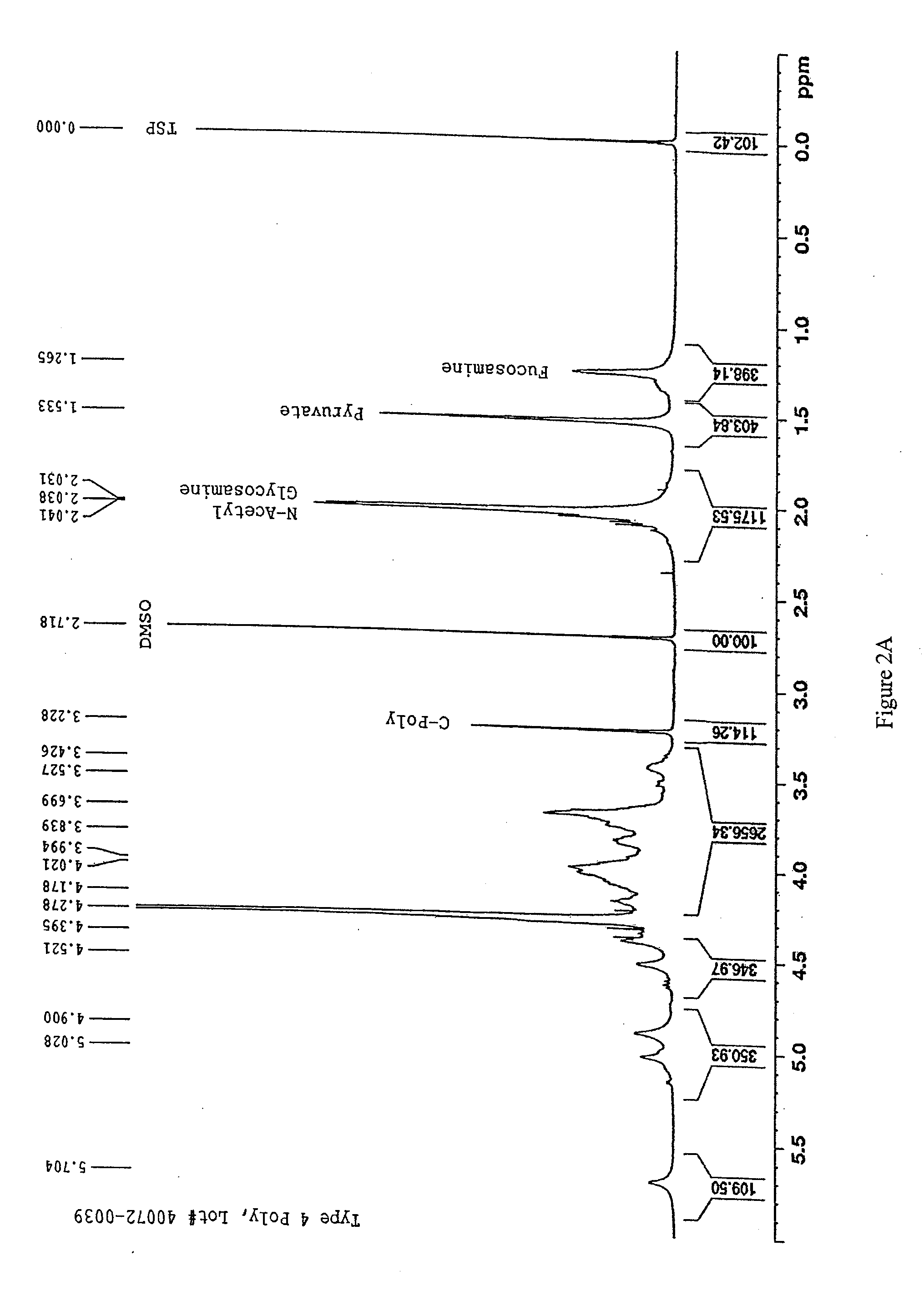
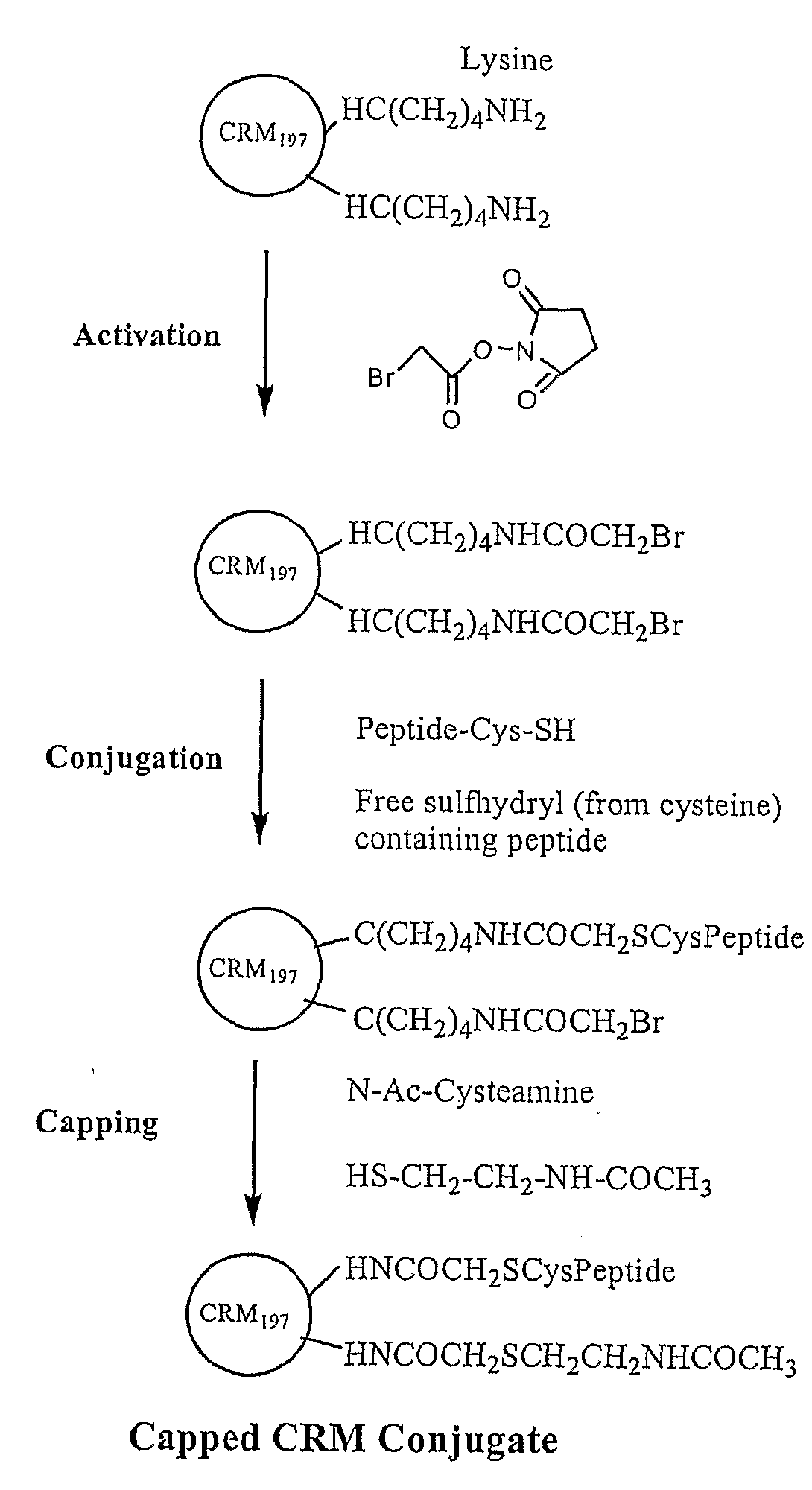
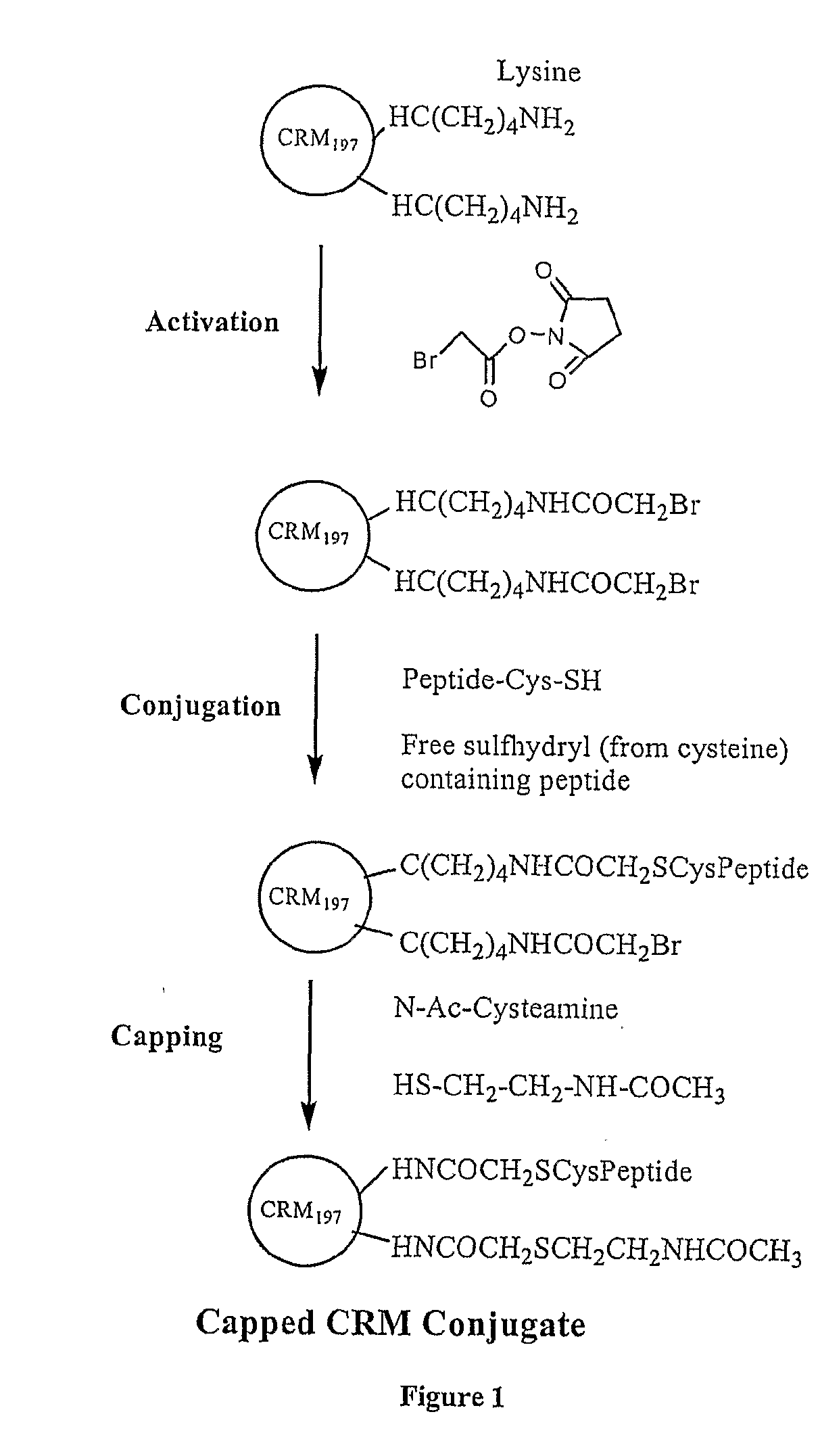
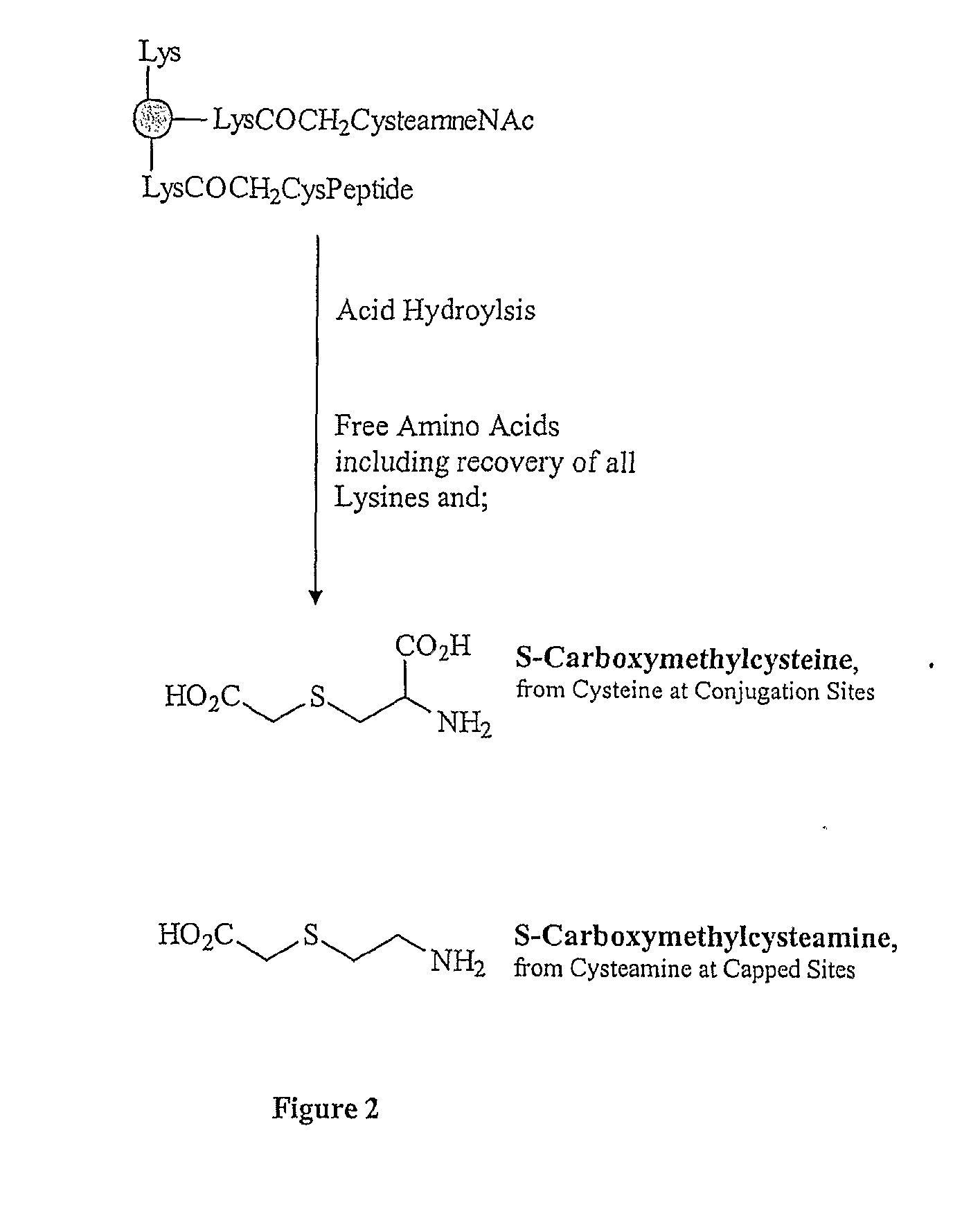
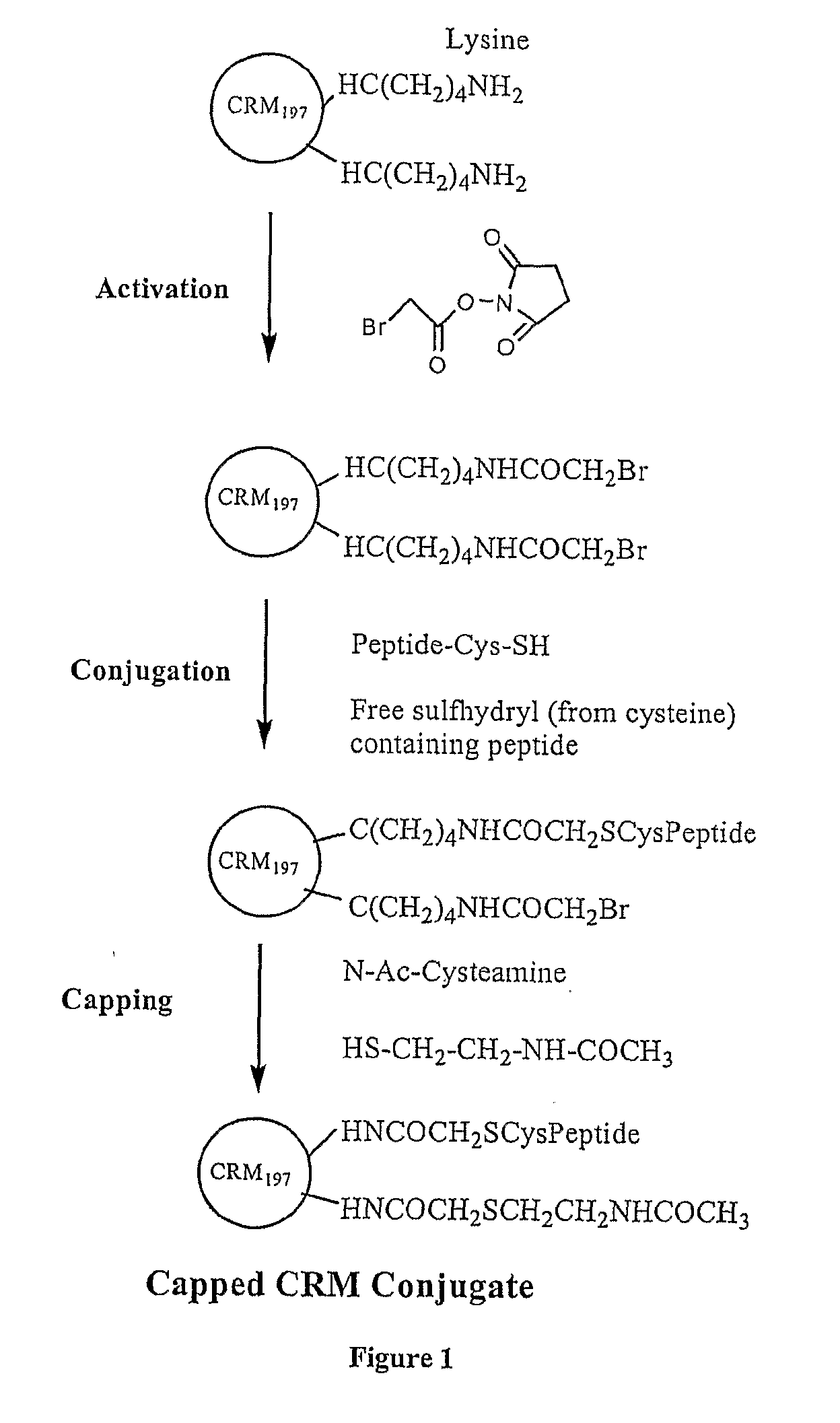
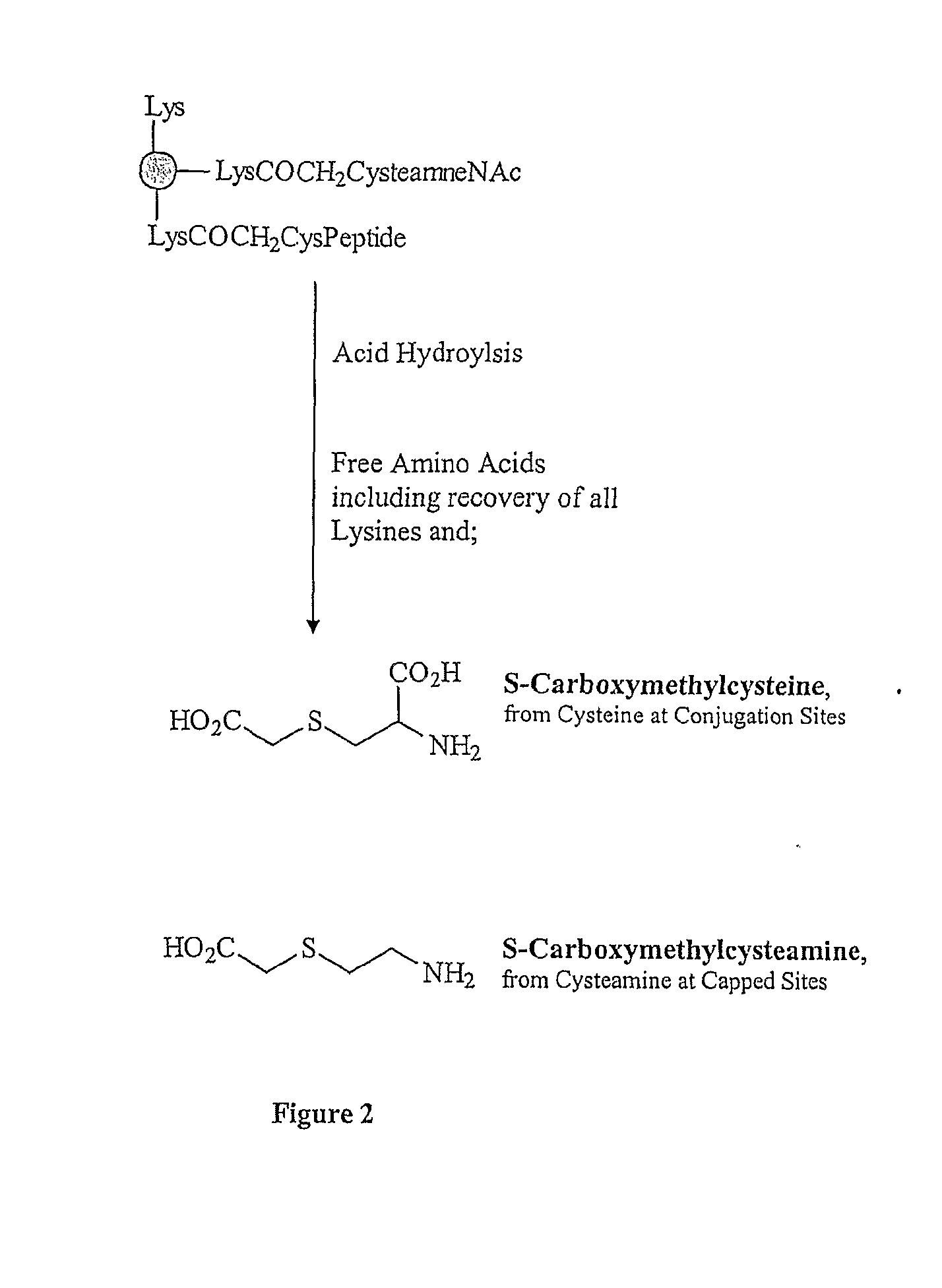
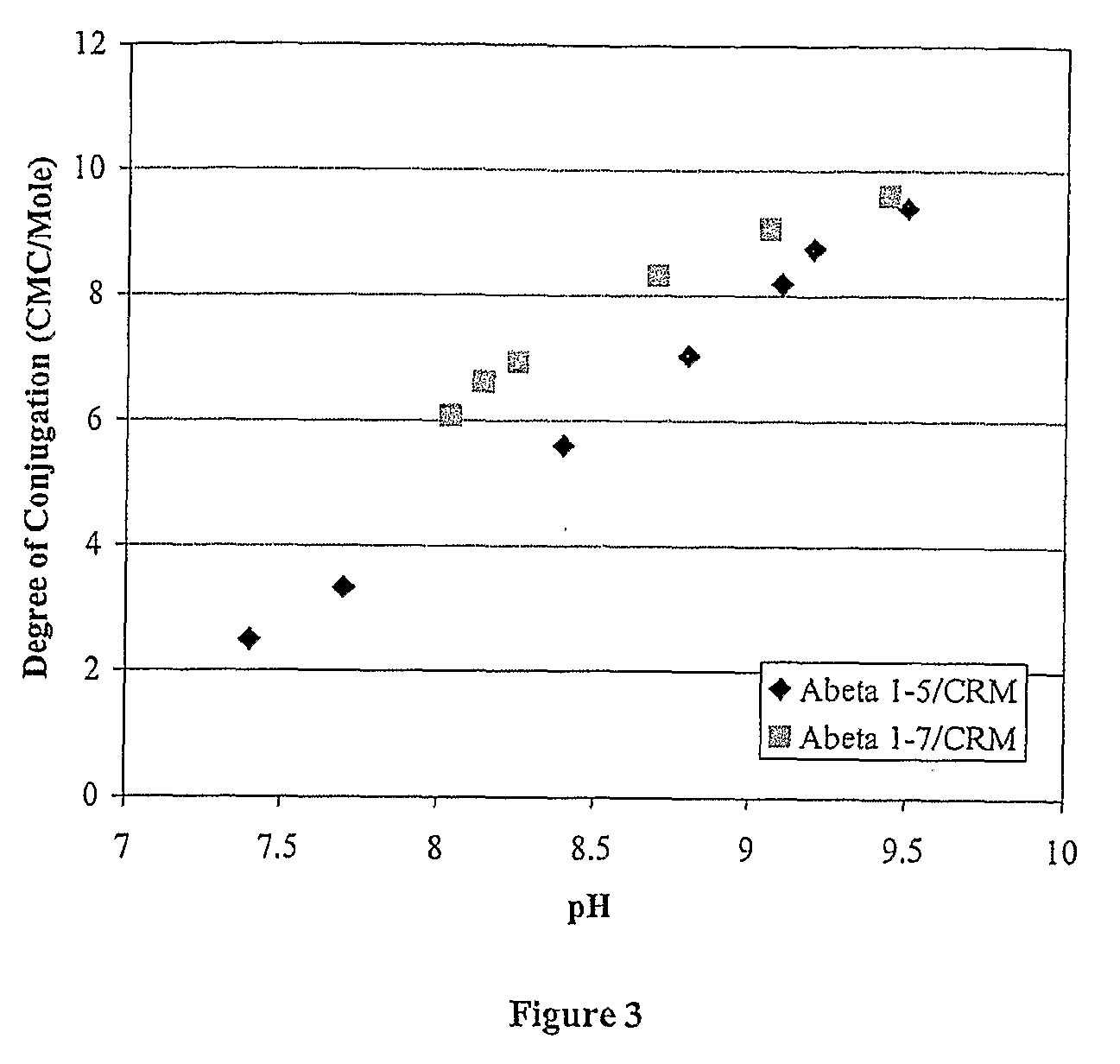
The present invention is directed to methods of producing conjugates of Aβ peptide immunogens with protein / polypeptide carrier molecules, which are useful as immunogens, wherein peptide immunogens are conjugated to protein carriers via activated functional groups on amino acid residues of the carrier or of the optionally attached linker molecule, and wherein any unconjugated reactive functional groups on amino acid residues are inactivated via capping, thus retaining the immunological functionality of the carrier molecule, but reducing the propensity for undesirable reactions that could render the conjugate less safe or effective. Furthermore, the invention also relates to such immunogenic products and immunogenic compositions containing such immunogenic products made by such methods.
Owner:JANSSEN SCI IRELAND UC +1
Process for preparing polysaccharide-protein conjugate vaccines
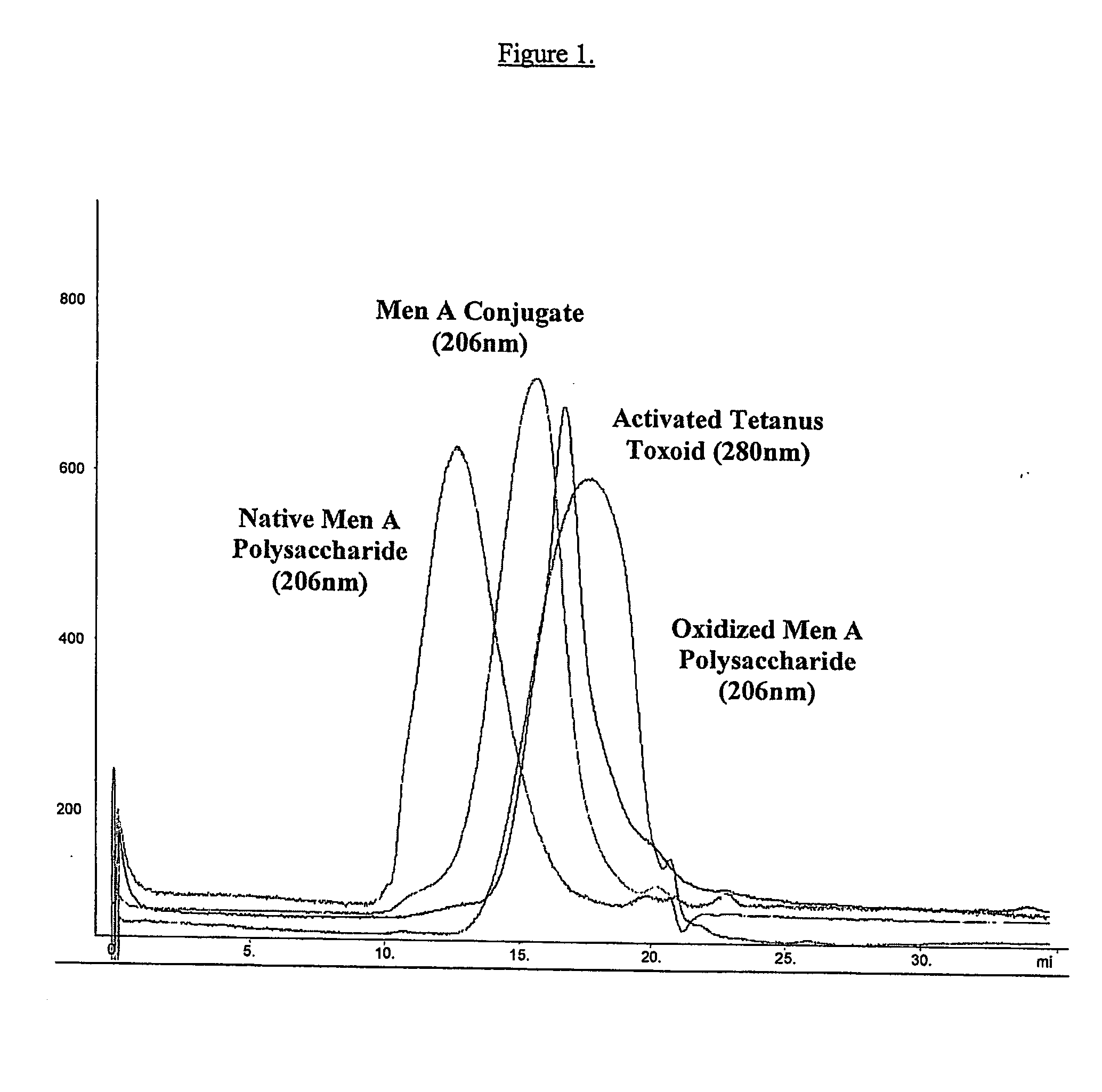
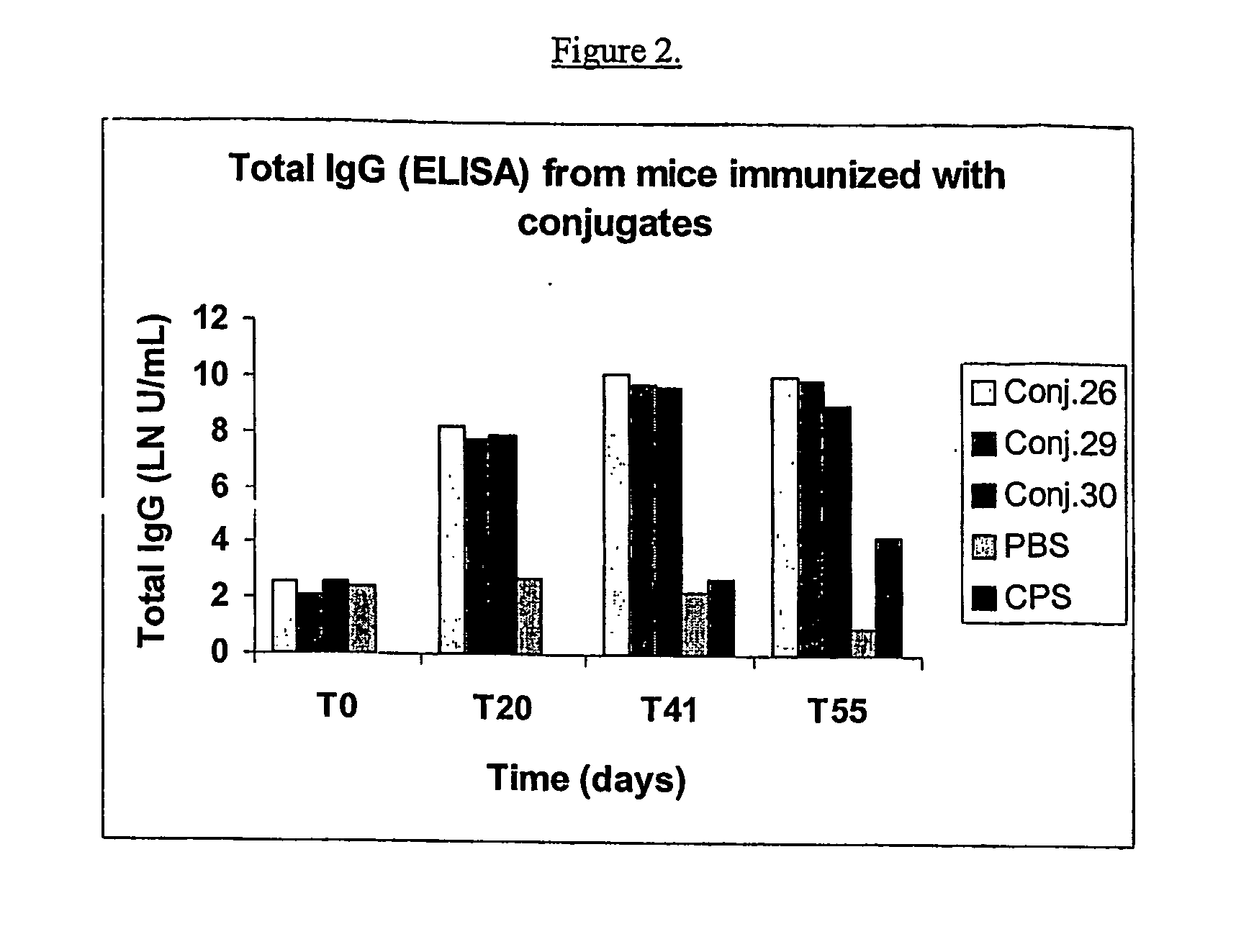
ActiveUS20070110762A1High yieldReduce productionAntibacterial agentsAntipyreticConjugate vaccineHydrazide
Methods for the manufacture of polysaccharide-protein conjugate vaccines at high yield are provided. The methods involve reaction of a hydrazide group on one reactant with an aldehyde group on the other reactant. The reaction proceeds rapidly with a high conjugation efficiency. Simplified purification processes can be employed to separate the conjugate product from the unconjugated protein and polysaccharide and other small molecule by-products.
Owner:DEPT OF HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC THE +1
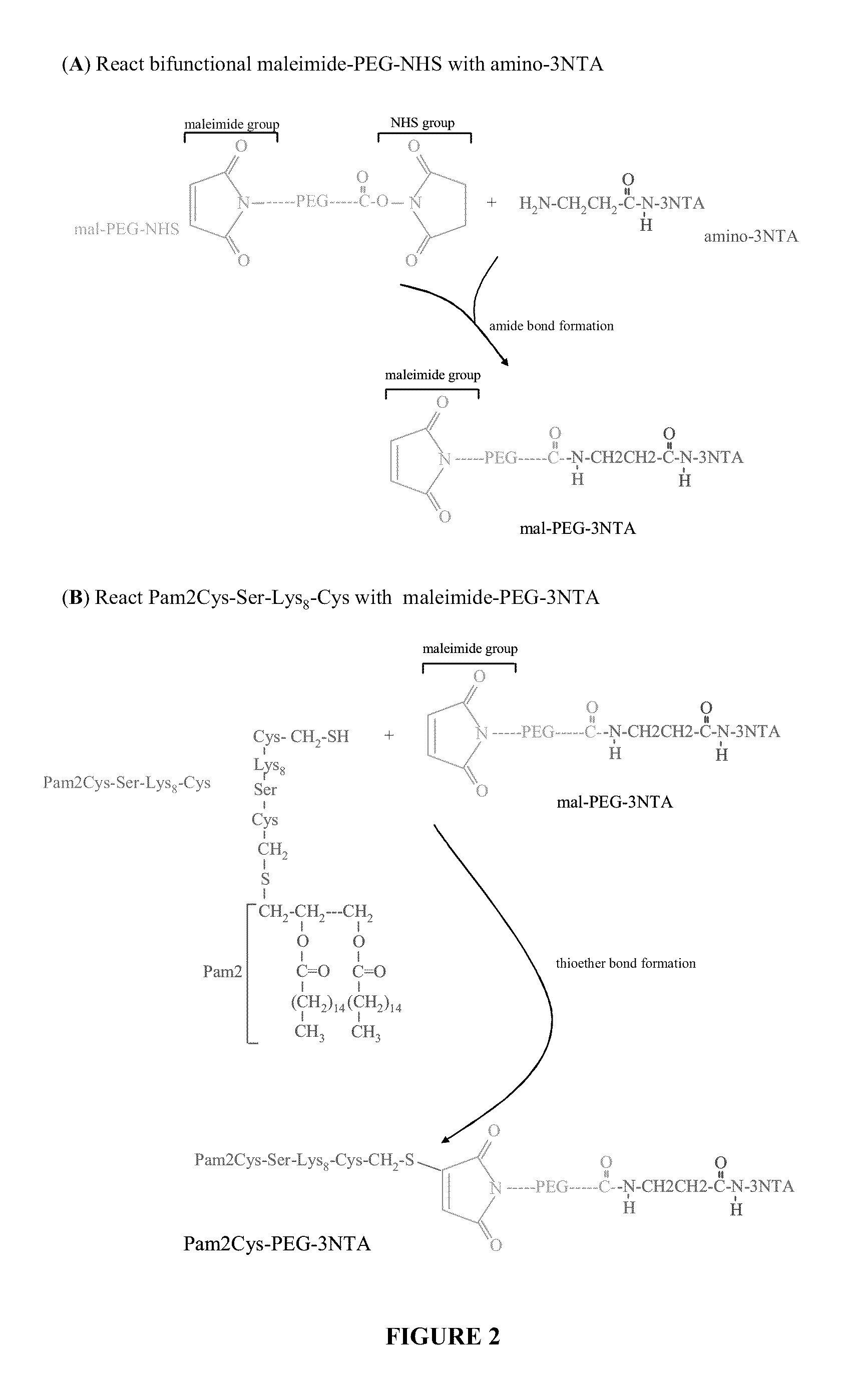
Adjuvanting Material
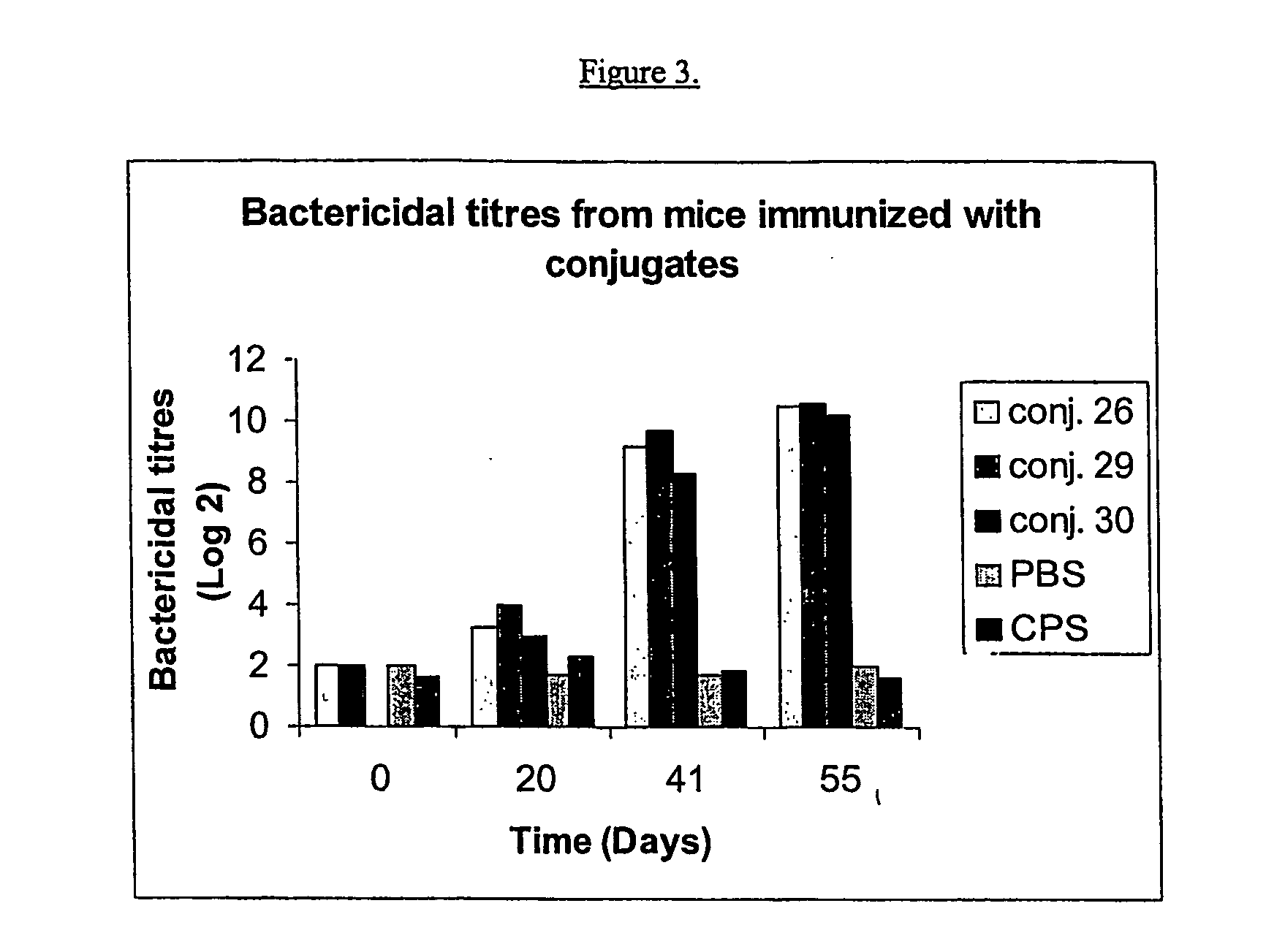
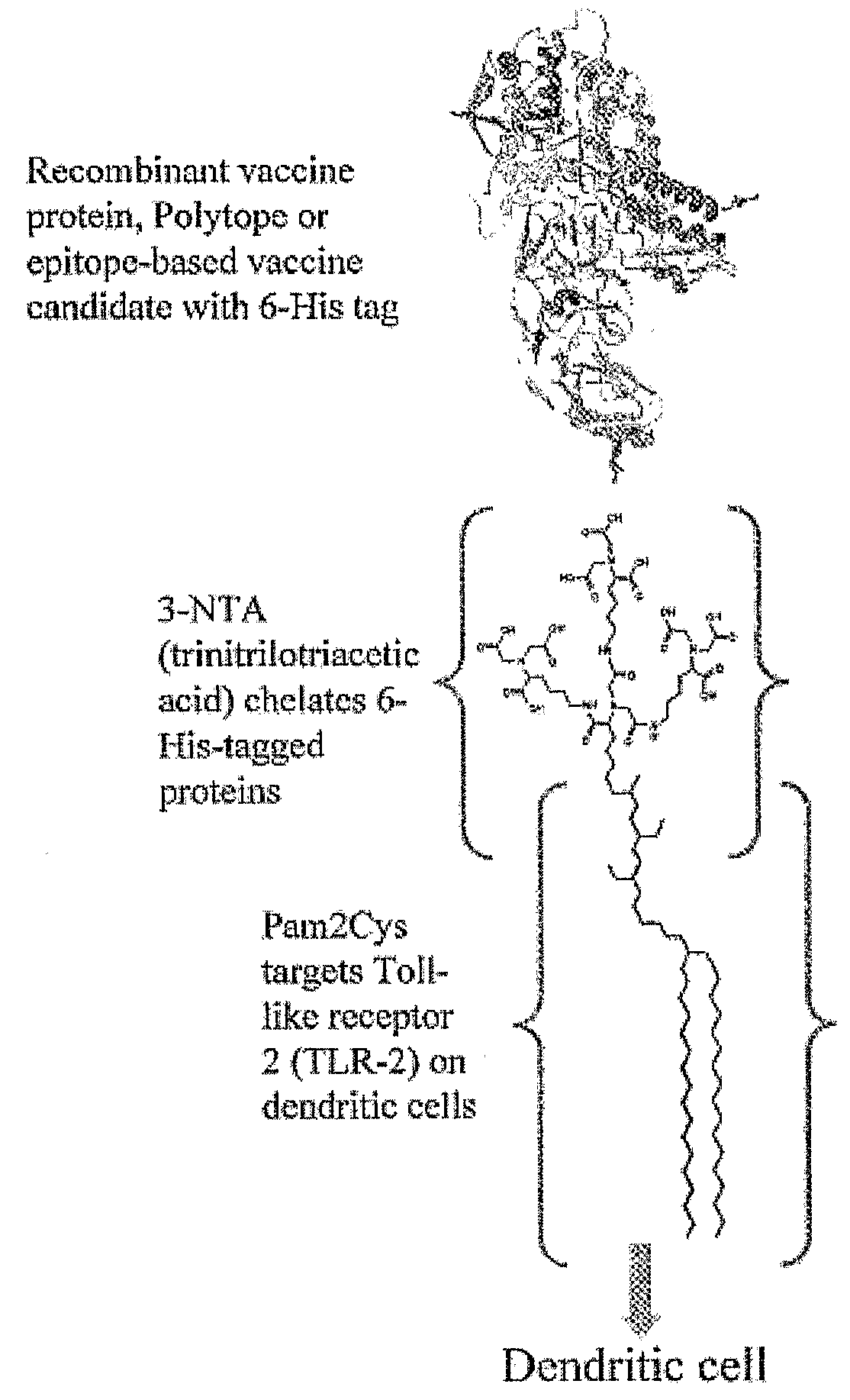
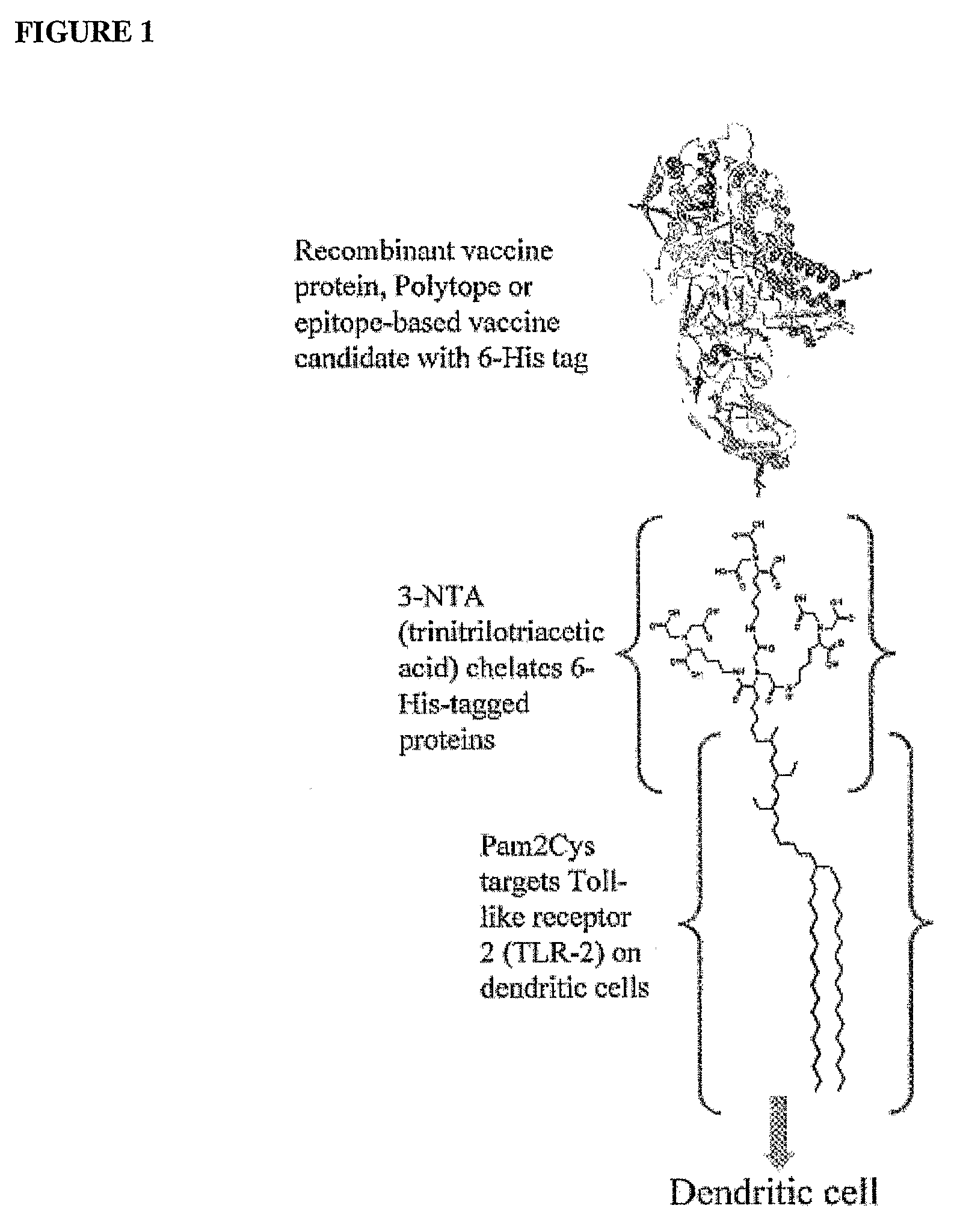
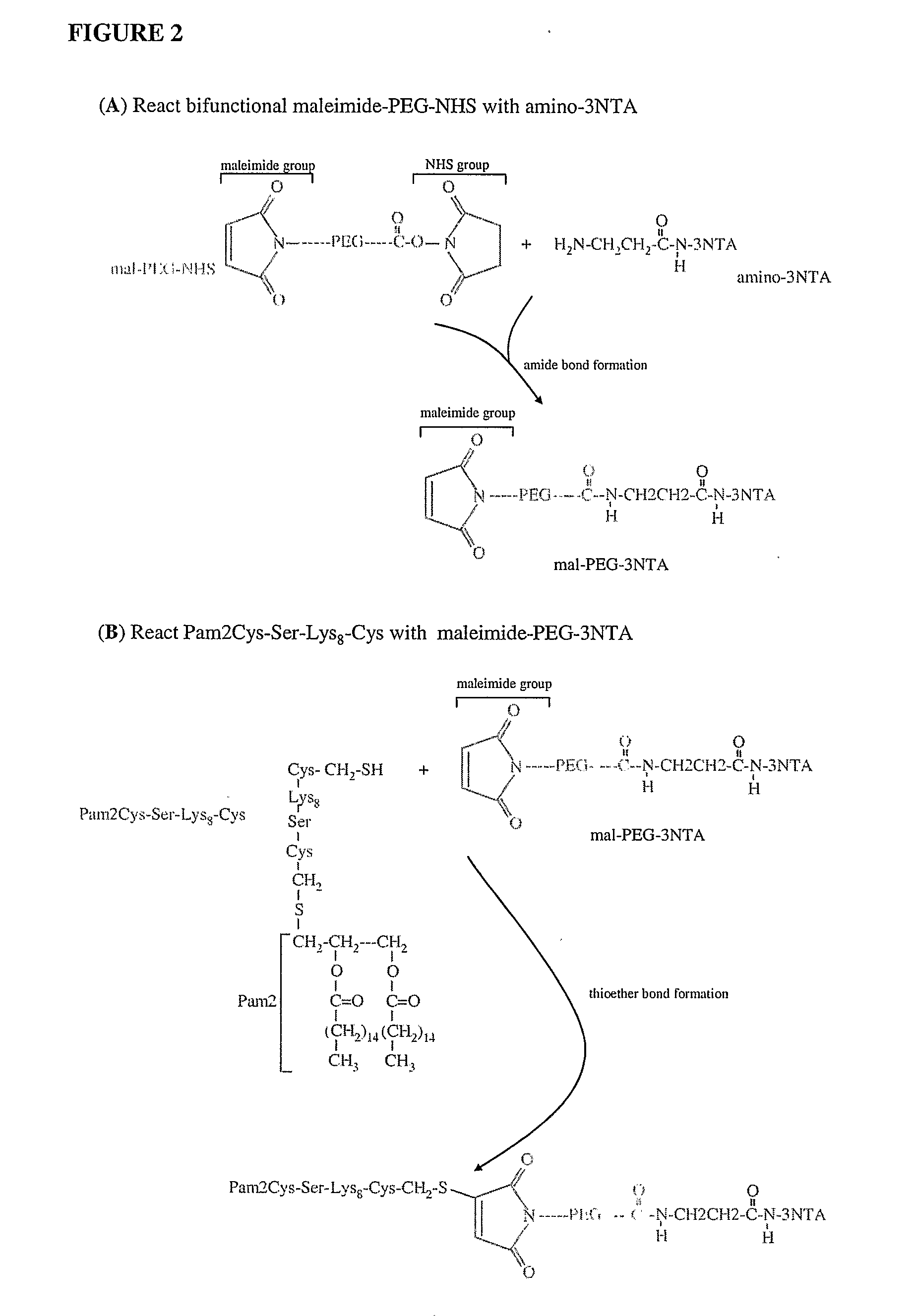
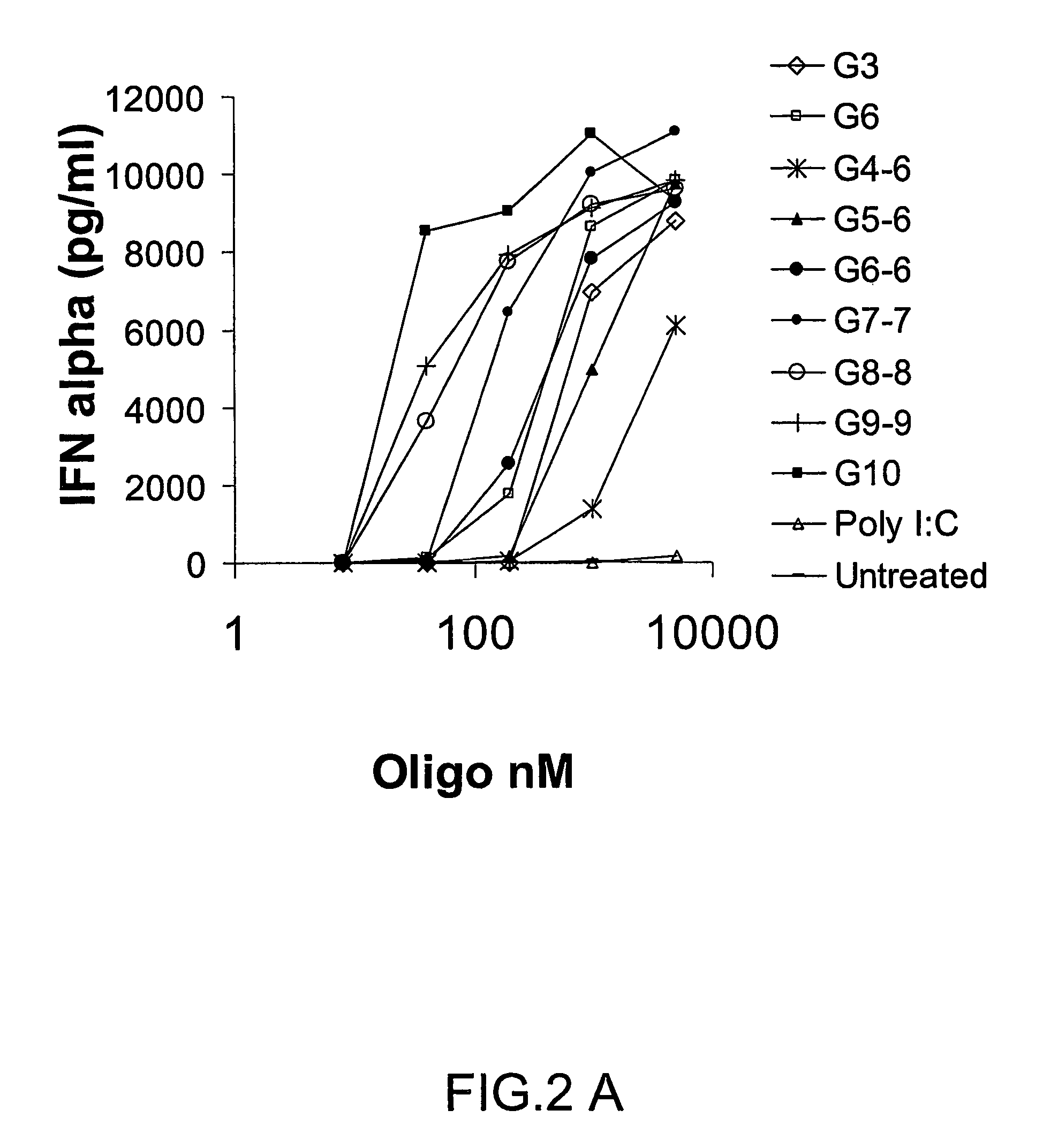
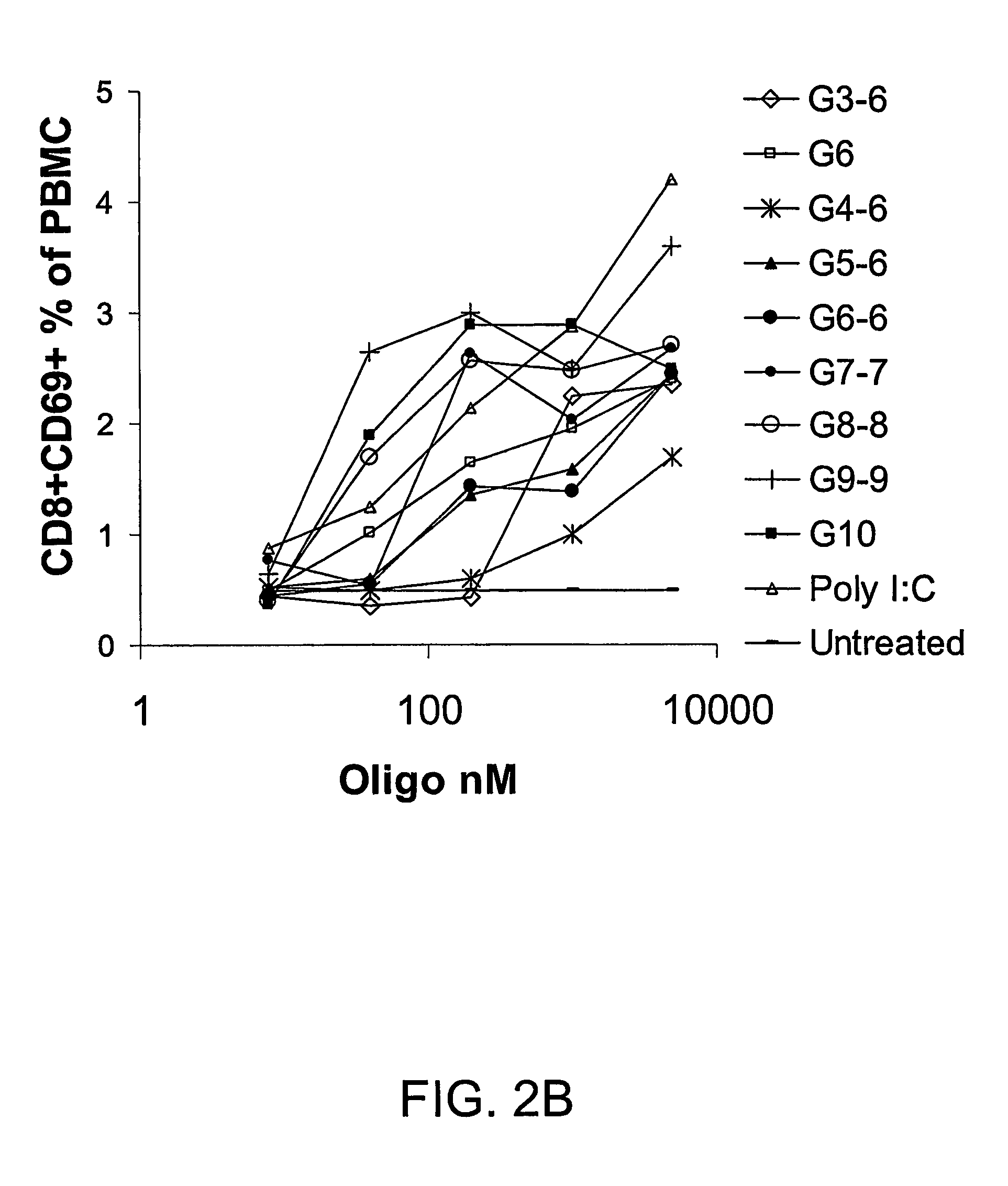
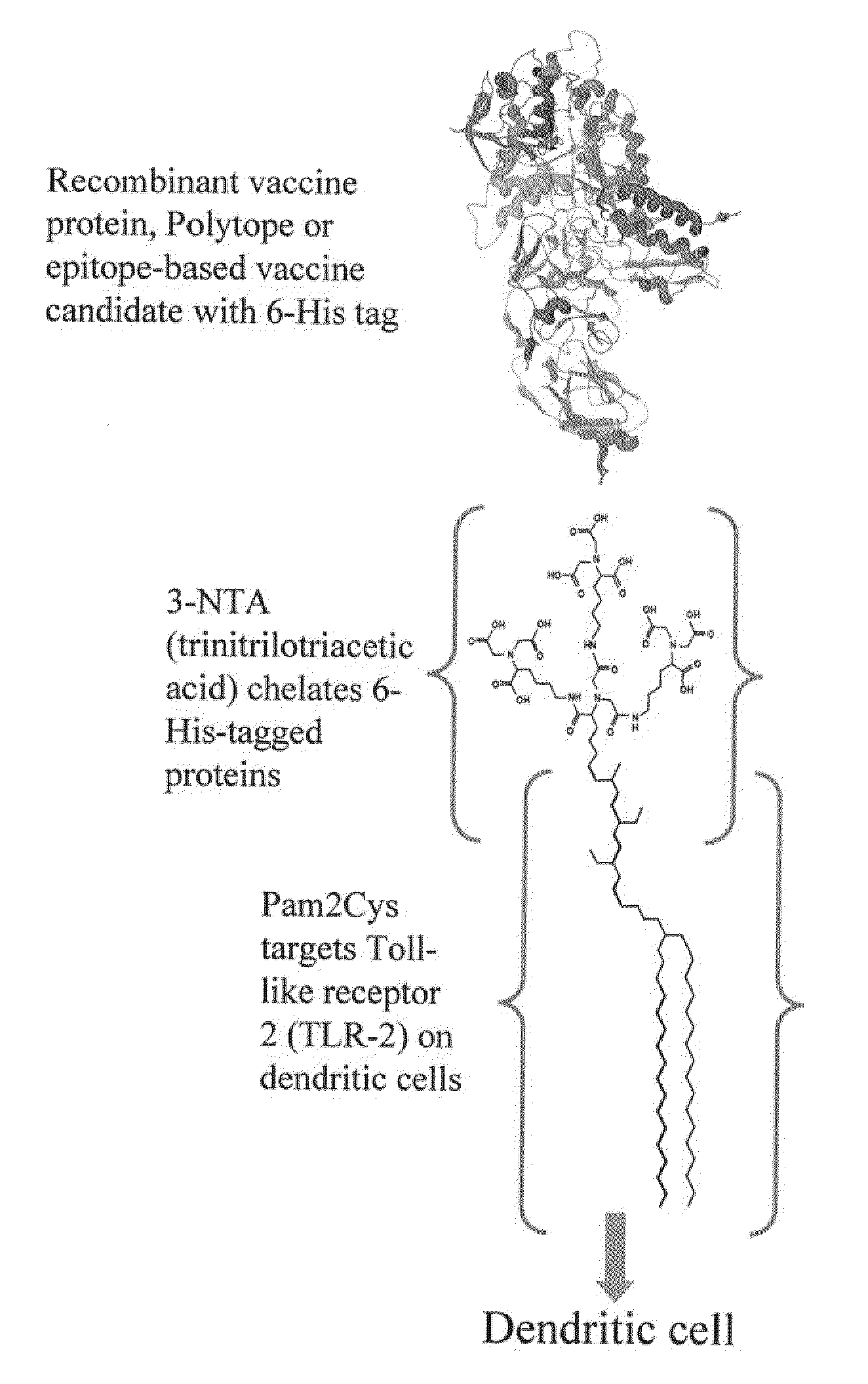
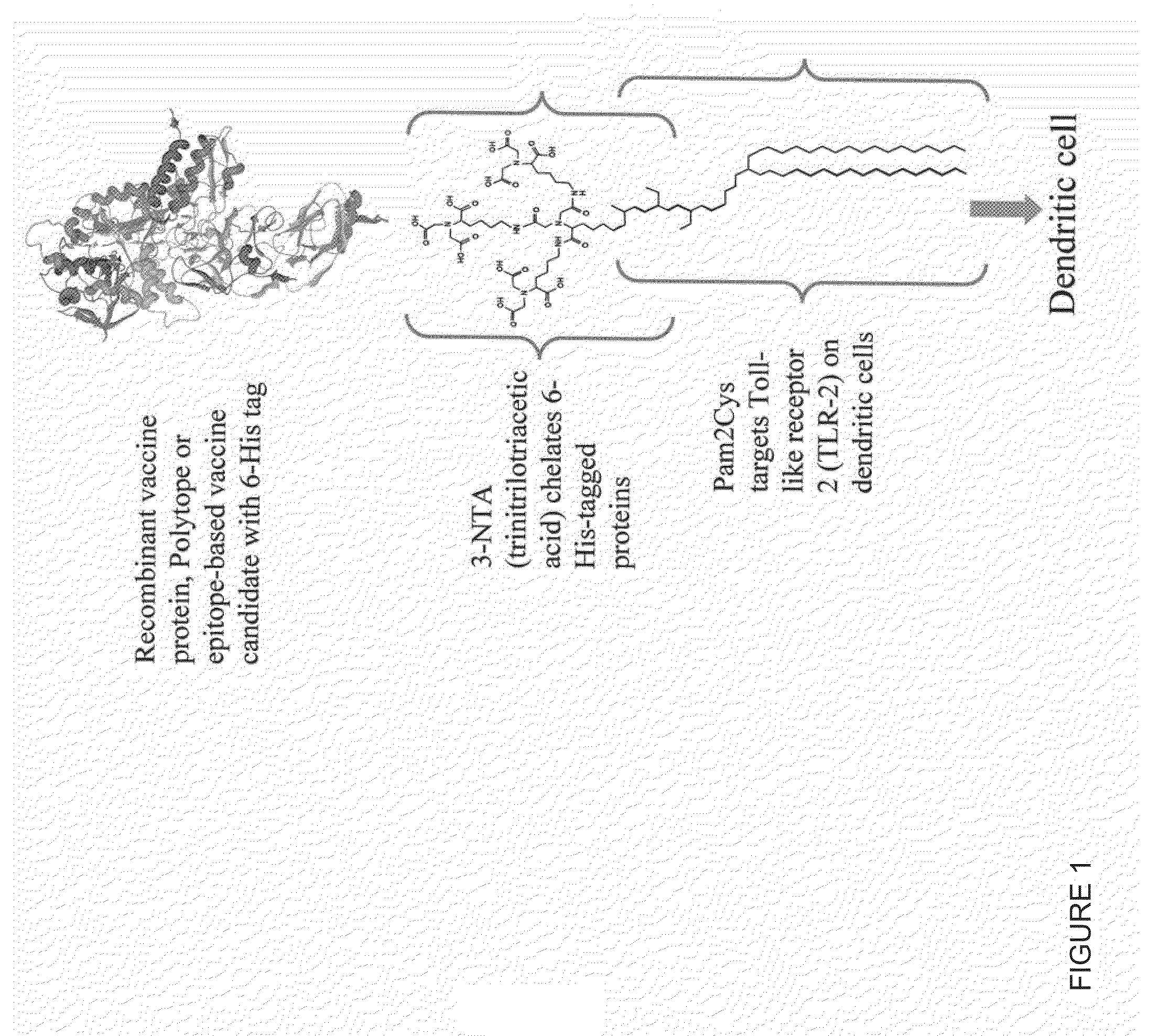
InactiveUS20080233143A1SsRNA viruses negative-senseOrganic active ingredientsLipid formationAdjuvant
The present invention provides an adjuvanting material, the adjuvanting material comprising a lipid dendritic cell targeting moiety to which is covalently linked a metal chelating group. Further, the present invention provides an immunogenic composition comprising (a) a lipid dendritic cell targeting moiety to which is covalently linked a metal chelating group; (b) an antigen comprising a metal affinity tag; and optionally (c) metal ions, whereby the antigen is linked to the lipid dendritic cell targeting moiety via the interaction between the metal affinity tag and the metal chelating group.
Owner:LIPOTEK PTY LTD
Vaccine Comprising Streptococcus Pneumoniae Capsular Polysaccharide Conjugates
InactiveUS20090017059A1Antibacterial agentsSenses disorderStreptococcus pneumoniae capsular polysaccharideStreptococcus mitis
The present invention discloses an immunogenic composition comprising S. pneumoniae capsular saccharide conjugates from serotypes 19A and 19F wherein 19A is conjugated to a first bacterial toxoid and 19F is conjugated to a second bacterial toxoid. Vaccines, methods of making vaccines and uses of the vaccines are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Melan-A- carrier conjugates
InactiveUS7537767B2Improve responseMore immunogenicSsRNA viruses negative-senseBiocideDiseaseAllergy
Owner:CYTOS BIOTECHNOLOGY AG
Adjuvanting material
The present invention provides an adjuvanting material, the adjuvanting material comprising a lipid dendritic cell targeting moiety to which is covalently linked a metal chelating group. Further, the present invention provides an immunogenic composition comprising (a) a lipid dendritic cell targeting moiety to which is covalently linked a metal chelating group; (b) an antigen comprising a metal affinity tag; and optionally (c) metal ions, whereby the antigen is linked to the lipid dendritic cell targeting moiety via the interaction between the metal affinity tag and the metal chelating group.
Owner:LIPOTEK PTY LTD
Vaccine
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
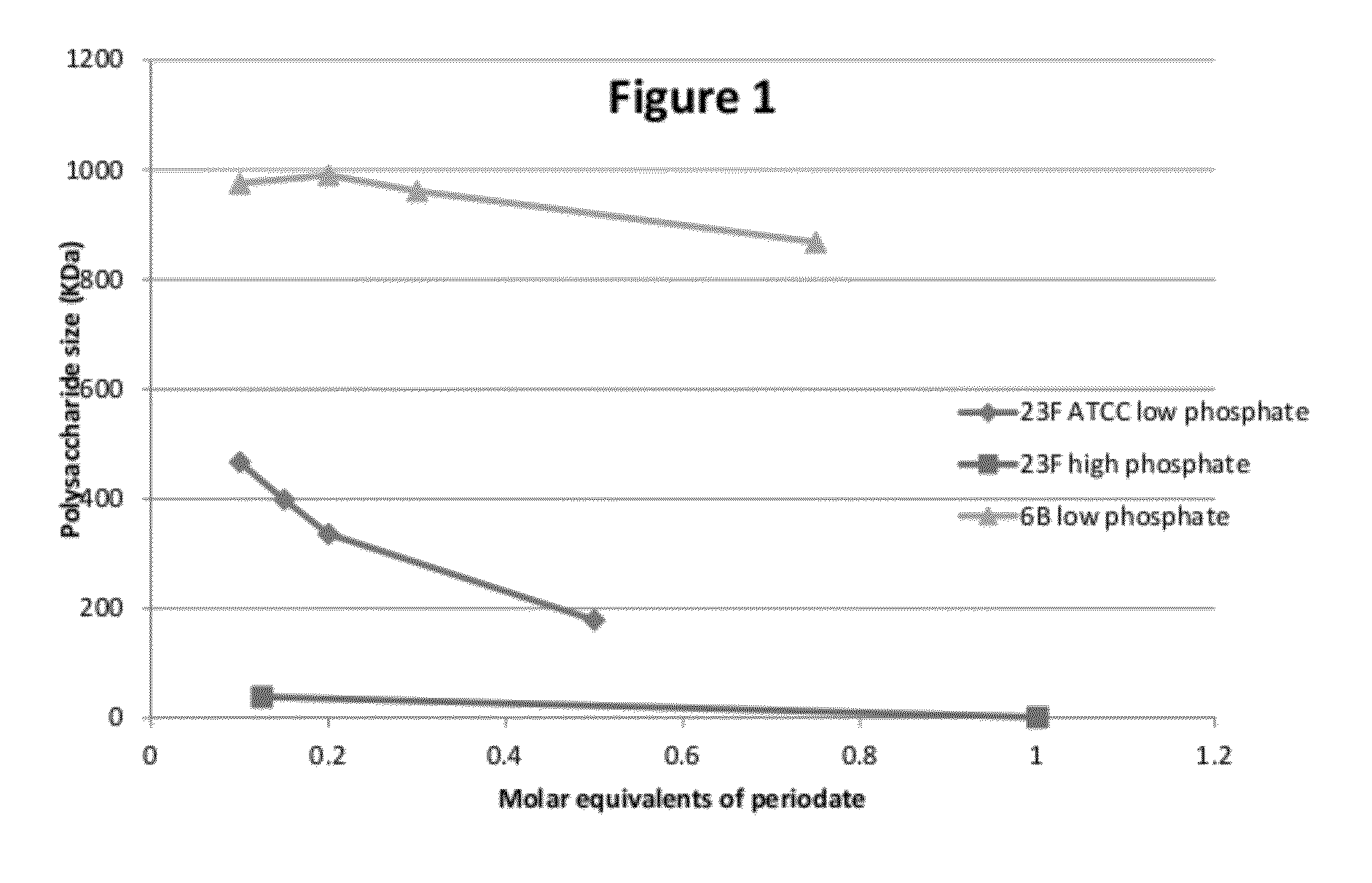
Conjugation process of bacterial polysaccharides to carrier proteins
ActiveUS8753645B2Antibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
Process for conjugation of bacterial saccharides including Streptococcus pneumoniae and Haemophilus influenzae saccharides by reductive amination are provided herein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
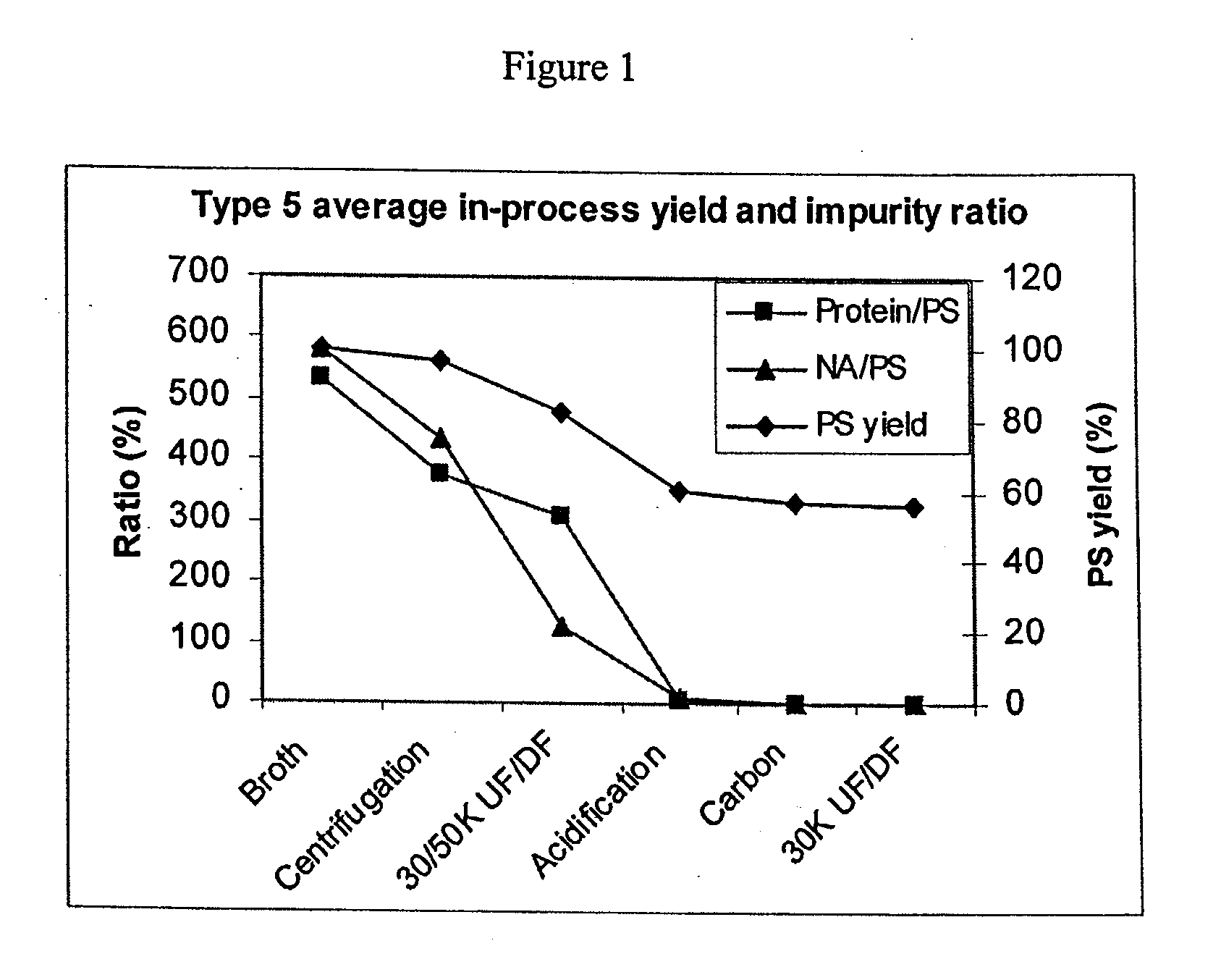
Shortened purification process for the production of capsular Streptococcus pneumoniae polysaccharides
ActiveUS8652480B2Reduce molecular weightImprove concentrationAntibacterial agentsBiocideActivated carbon filtrationSarcosine
A shortened process for producing a solution containing substantially purified capsular polysaccharides from a cellular Streptococcus pneumoniae lysate broth is described. Ultrafiltering and diafiltering a clarified S. pneumoniae lysate followed by pH adjustment to less than 4.5, preferably about 3.5, precipitated at least 98% of the protein in the solution without seriously affecting polysaccharide yield. Furthermore, following ultrafiltration and diafiltration and acidification to a pH of less than 4.5, filtration using activated carbon precipitated at least 90% of remaining protein without seriously affecting polysaccharide yield. Exemplary, non-limiting S. pneumoniae serotypes that can be purified using the shortened process of the invention are 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. In one embodiment, the Streptococcus pneumoniae cells are lysed using deoxycholate sodium (DOC), while in another embodiment the lytic agent is a non-animal derived lytic agent such as N-lauryl sarcosine sodium (NLS).
Owner:WYETH LLC
Melan-a peptide analogue-virus-like-particle conjugates
InactiveUS20060204475A1More immunogenicImprove responseSsRNA viruses negative-senseBiocideAbnormal tissue growthViral disease
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a modified virus-like particle (VLP) comprising a VLP which can be loaded with immunostimulatory substances, in particular with DNA oligonucleotides containing non-methylated C and G (CpGs), and particular peptides derived from MelanA linked thereto. Such CpGVLPs are dramatically more immunogenic than their CpG-free counterparts and induce enhanced B and T cell responses. The immune response against MelanA peptide analogues optionally coupled, fused or attached otherwise to the VLPs is similarly enhanced as the immune response against the VLP itself. In addition, the T cell responses against the MelanA peptide analogues are especially directed to the Thl type. Antigens attached to CpG-loaded VLPs may therefore be ideal vaccines for prophylactic or therapeutic vaccination against allergies, tumors and other self-molecules and chronic viral diseases.
Owner:CYTOS BIOTECHNOLOGY AG
Stable immunogenic product comprising antigenic heterocomplexes
ActiveUS20060013800A1Induce productionPeptide/protein ingredientsVertebrate antigen ingredientsImmunogenicityAntigenic protein
A stable immunogenic product for the induction of antibodies against one or more antigenic proteins in a subject, characterized in that it comprises proteinaceous immunogenic heterocomplexes which are formed by associations between (i) antigenic protein molecules and (ii) proteinaceous carrier molecules and in that less than 40% of the antigenic proteins (i) are linked to the proteinaceous carrier molecules (ii) by a covalent bond.
Owner:NEOVACS SA
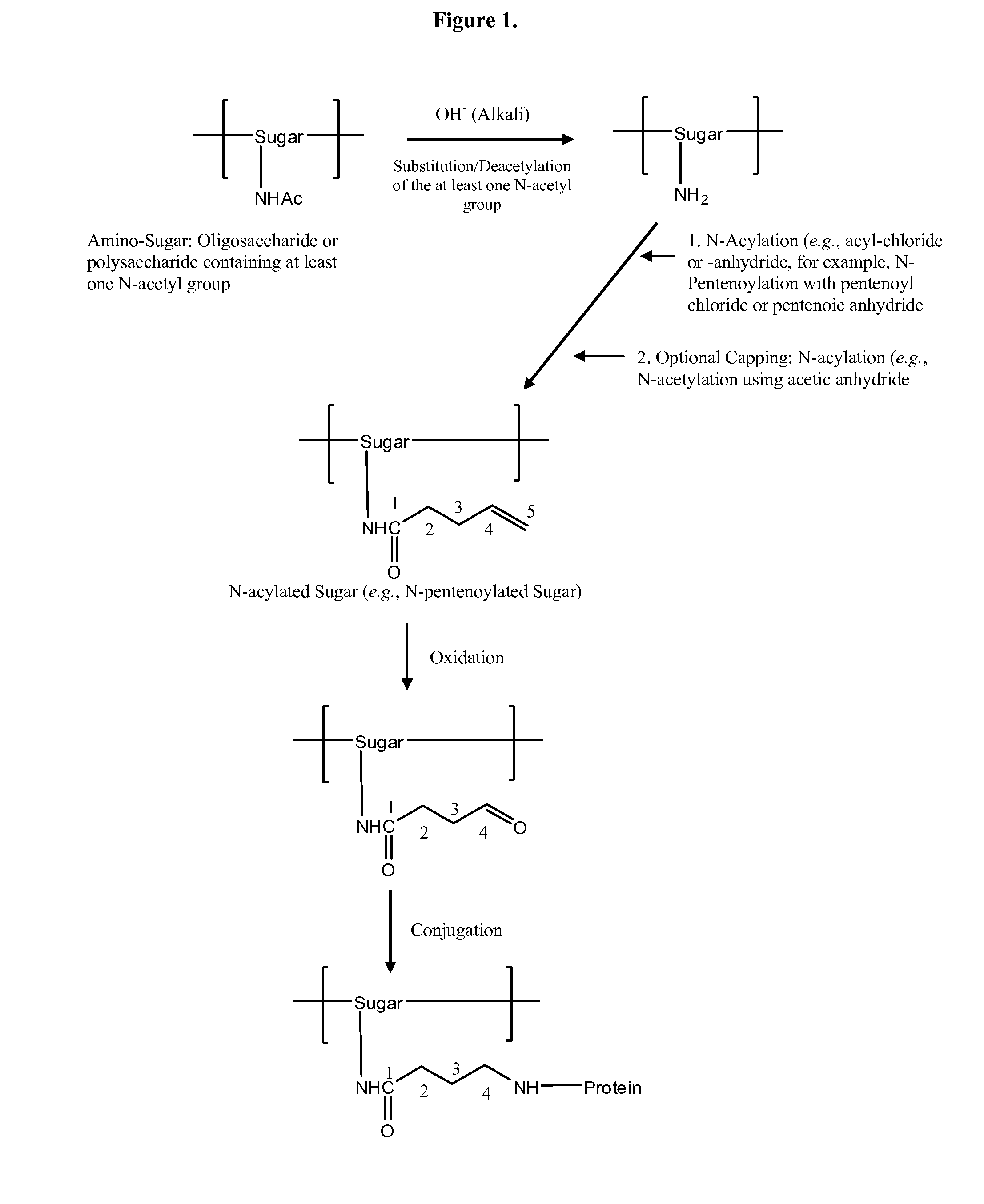
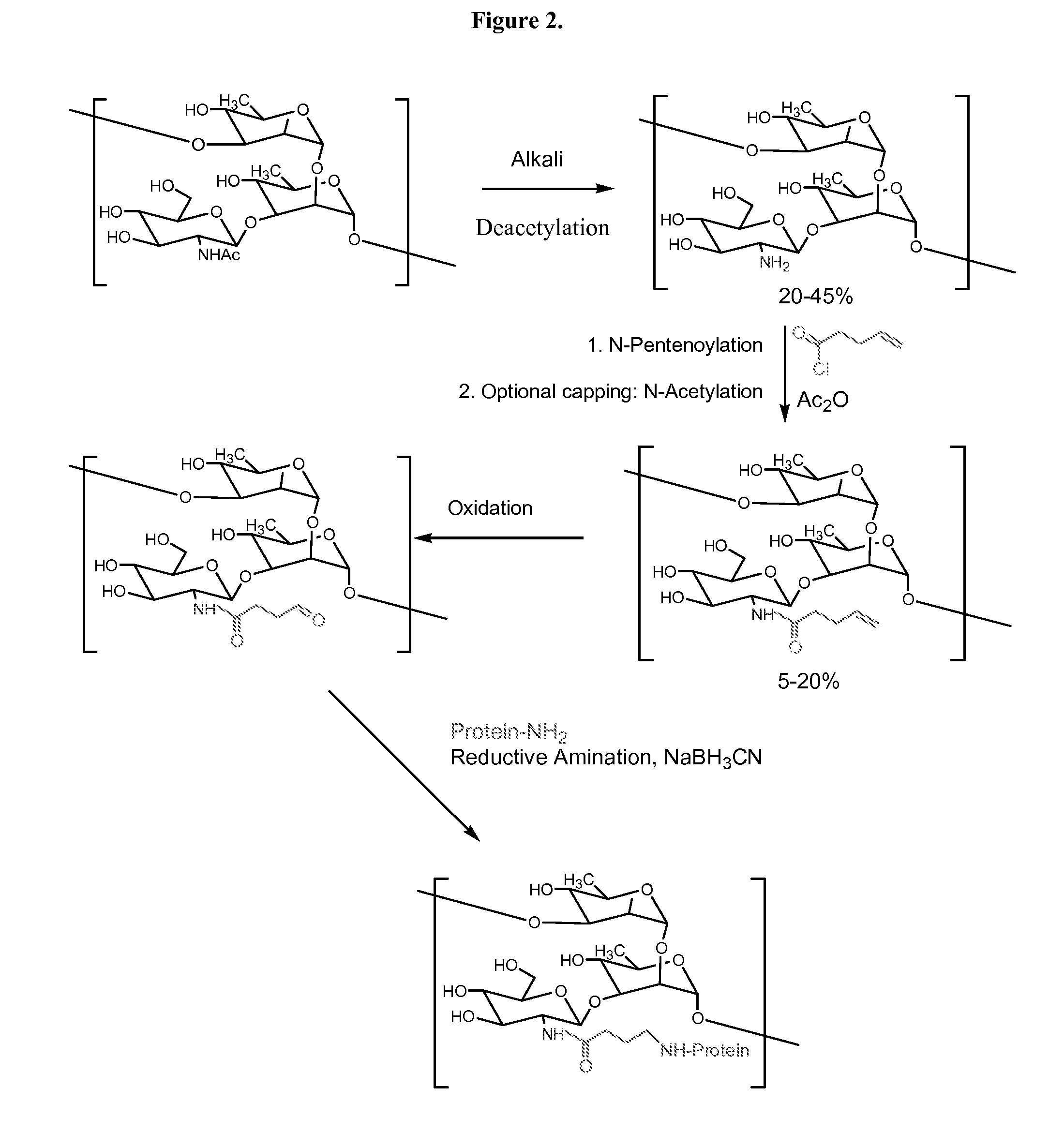
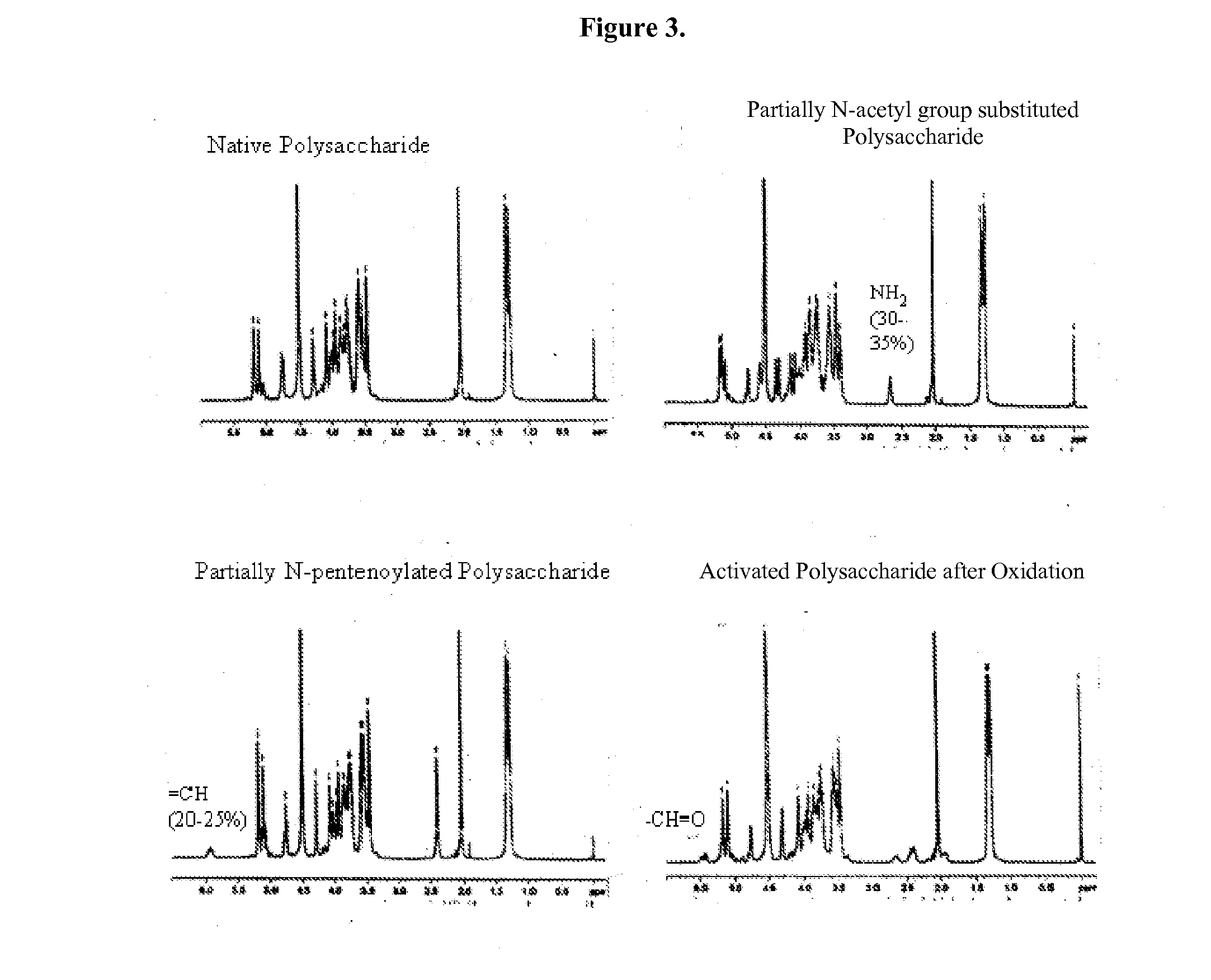
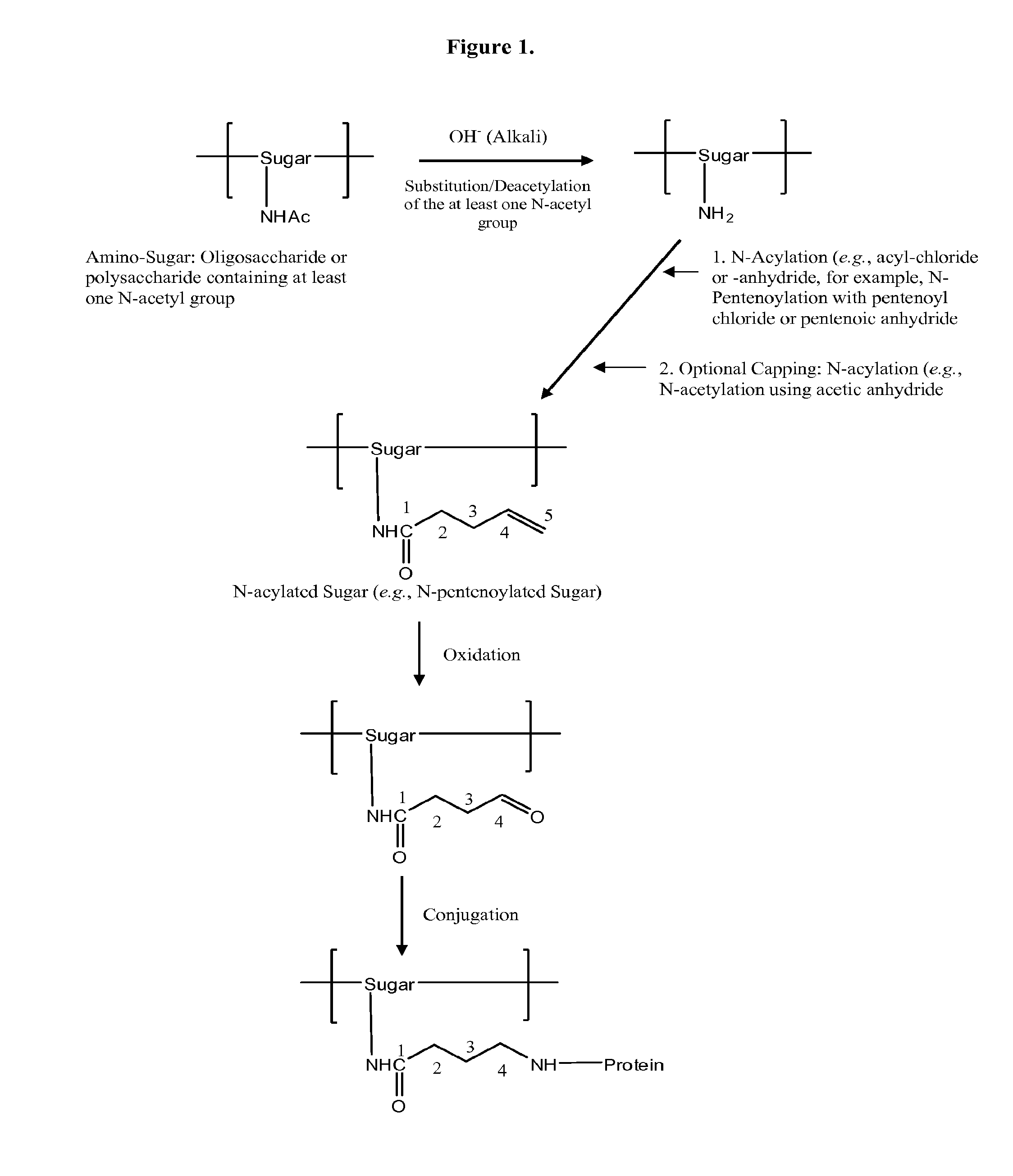
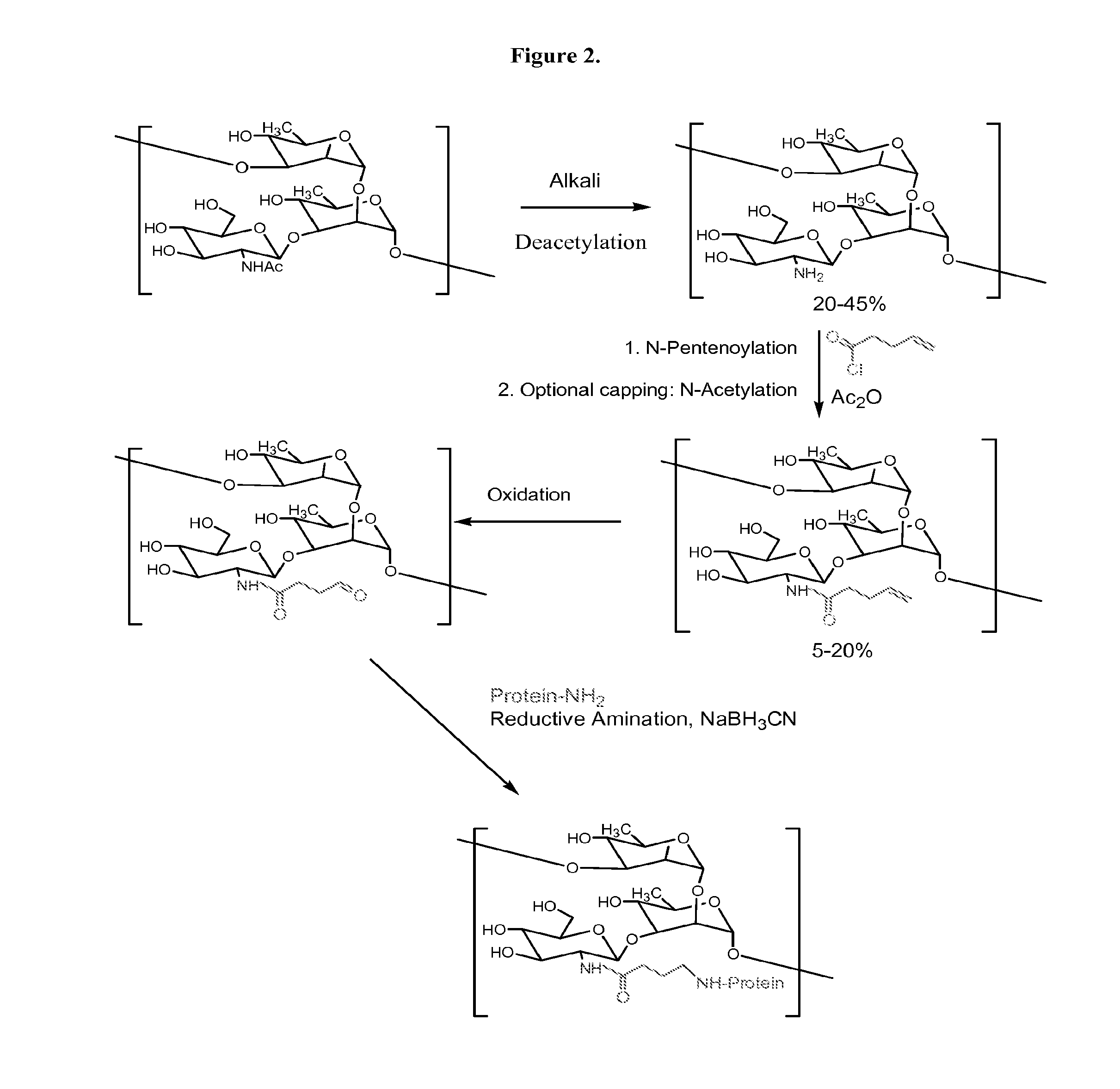
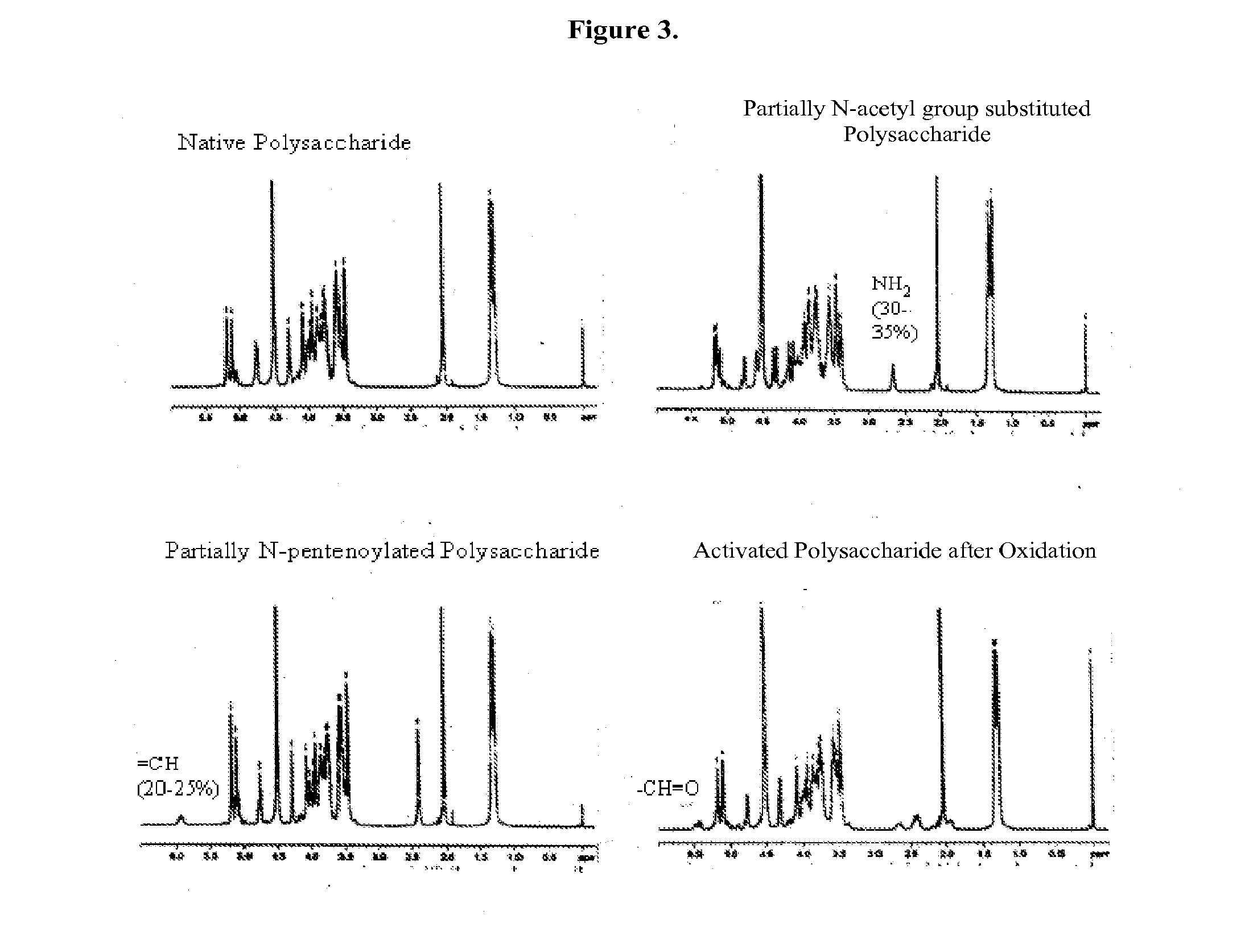
Modified polysaccharides for conjugate vaccines
InactiveUS20100189740A1Good antigenicityContribute to the valency of the vaccineAntibacterial agentsDepsipeptidesConjugate vaccineMicroorganism
The present invention relates to methods of manufacture of immunogenic glycoconjugates, in particular for use in pharmaceutical compositions for inducing a therapeutic immune response in a subject. The immunogenic glycoconjugates of the invention comprise one or more oligosaccharides or polysaccharides that are conjugated to one or more carrier proteins via an active aldehyde group. Accordingly, the invention provides methods of making (i) unsaturated microbial N-acyl derivative oligosaccharides or polysaccharides; (ii) novel conjugates of unsaturated N-acyl derivatives; and (iii) glycoconjugate compositions comprising conjugate molecules of fragments of microbial unsaturated N-acyl derivatives that serve as a covalent linker to one or more proteins. The invention further encompasses the use of the immunogenic glycoconjugates pharmaceutical compositions for the prevention or treatment of an infectious disease.
Owner:PFIZER IRELAND PHARM CORP
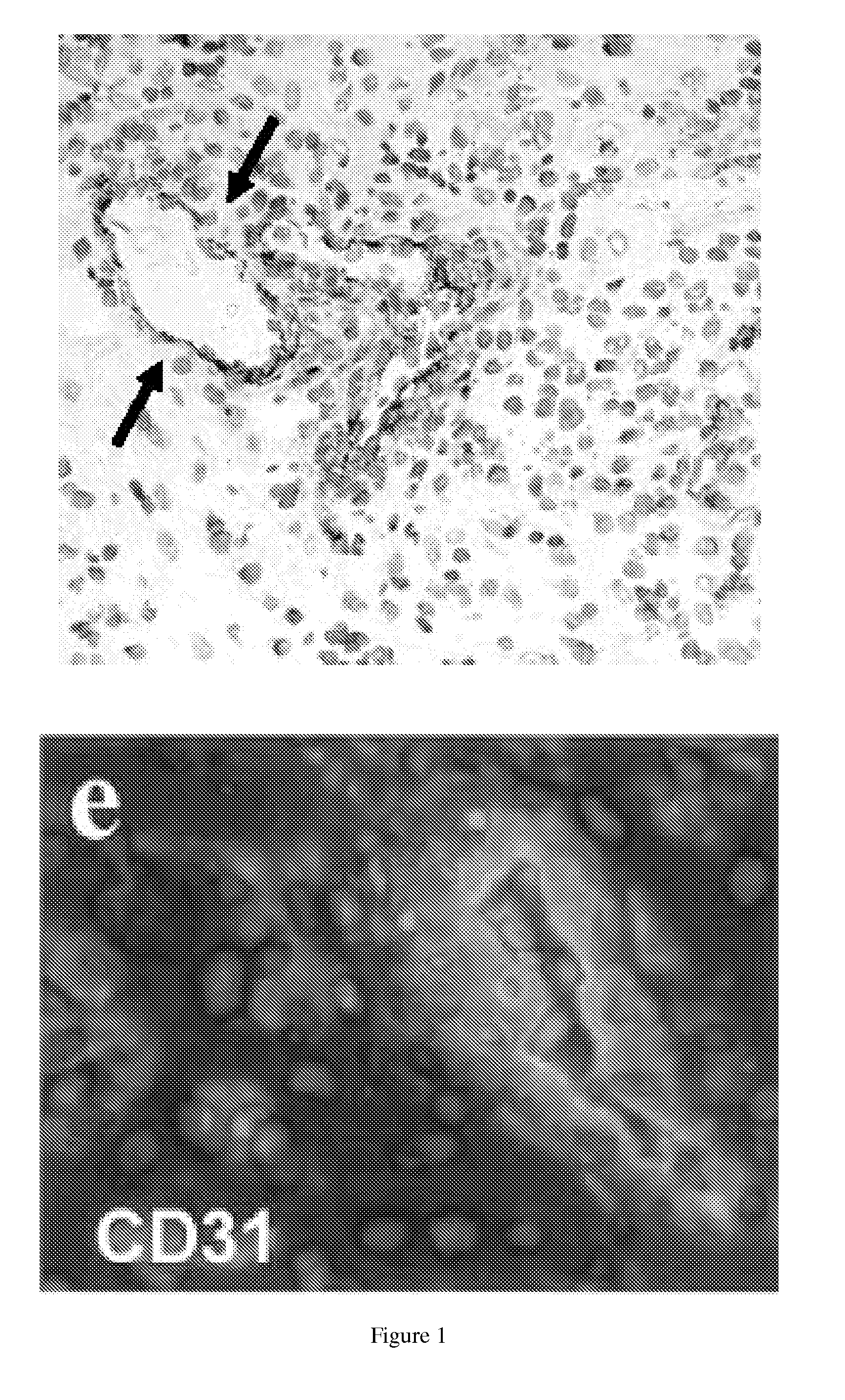
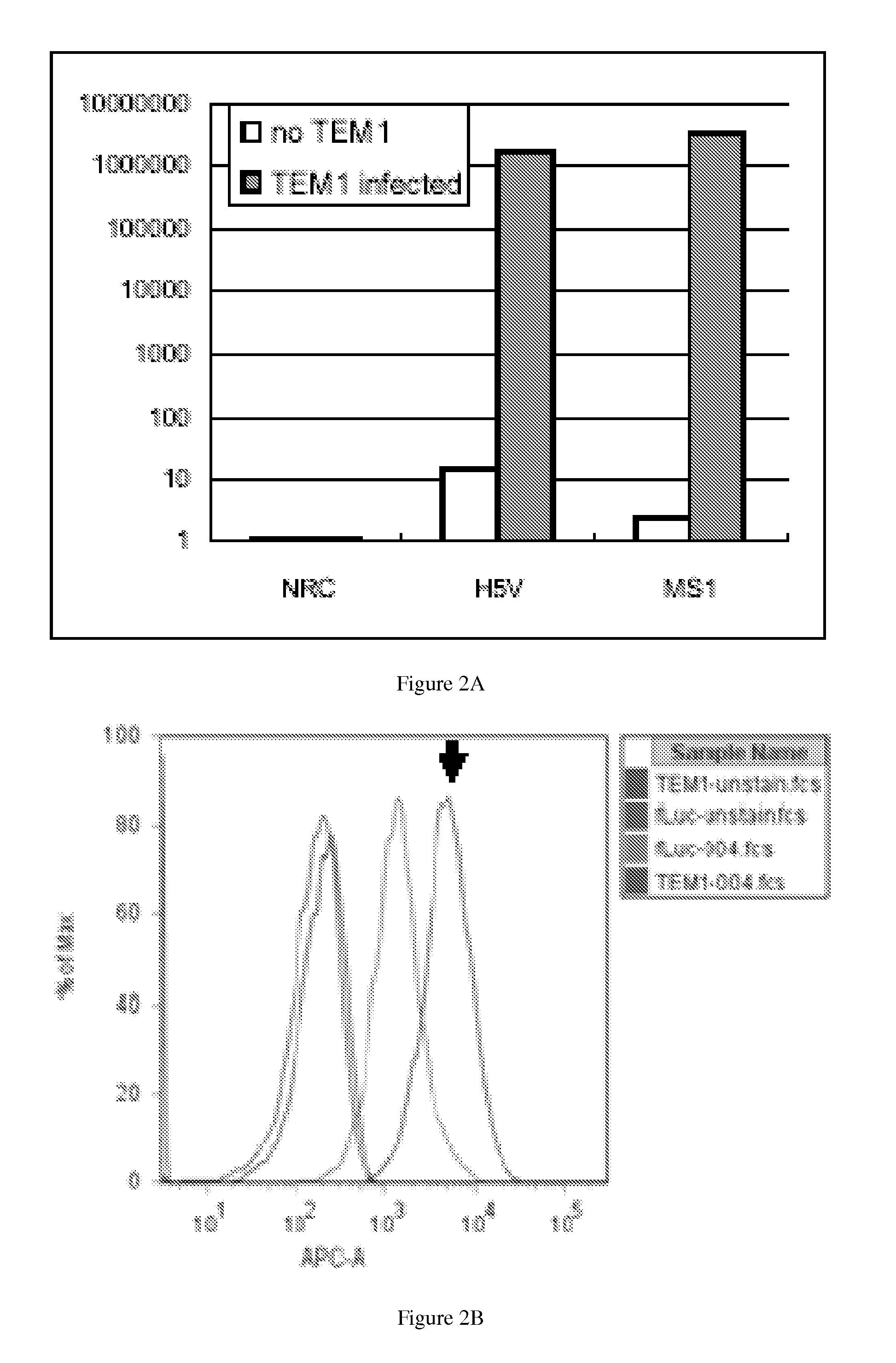
Tumor vascular marker-targeted vaccines
ActiveUS20120035529A1Prevent relapseElectrotherapyTumor rejection antigen precursorsTumor recurrenceTumor vessel
The present invention provides methods of immunizing a subject against a tumor, inhibiting tumor growth, inhibiting tumor recurrence, treating, suppressing the growth of, or decreasing the incidence of a tumor, overcoming tolerance to a tumor vasculature marker (TVM) in a subject comprising the step of administering a vaccine comprising a TVM or a nucleic acid encoding a TVM and related vaccines. The present invention also provides a method of targeting a tumor vasculature in a subject having a tumor comprising the step of contacting said subject with a labeled compound that binds a) a tumor vasculature marker (TVM) or b) a nucleic acid molecule encoding said TVM.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
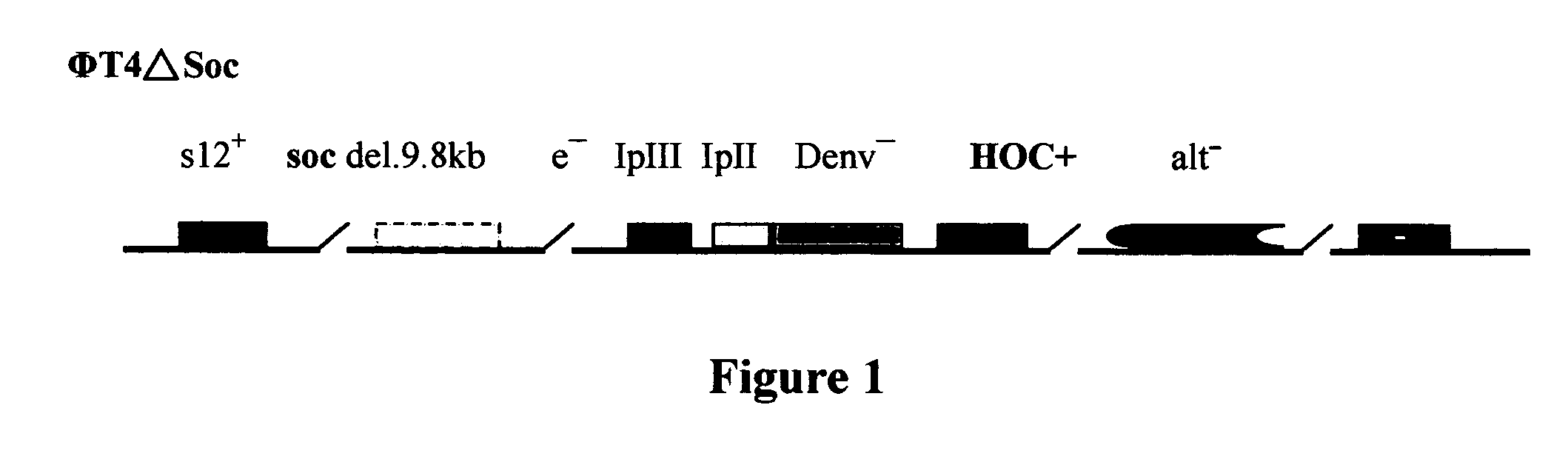
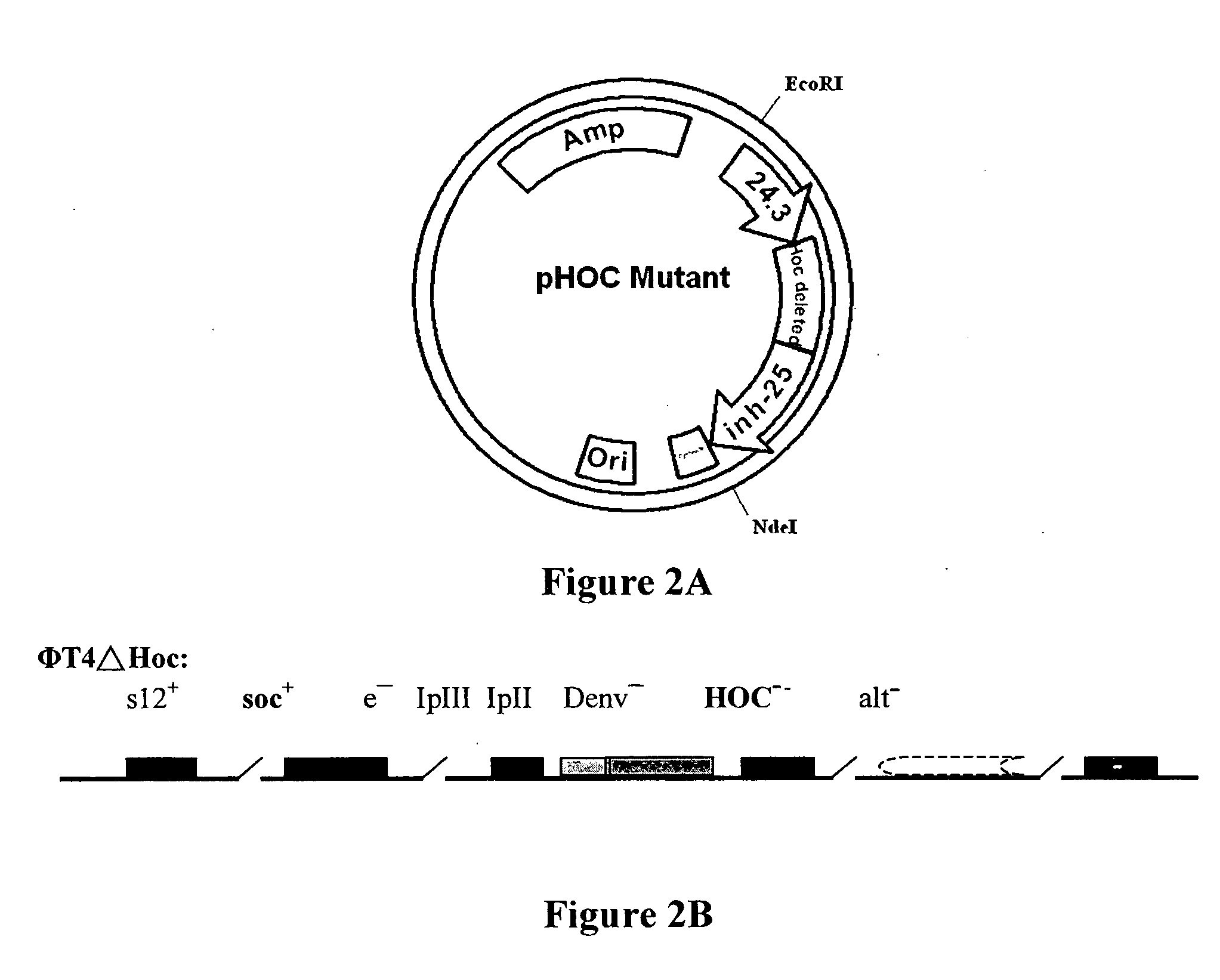
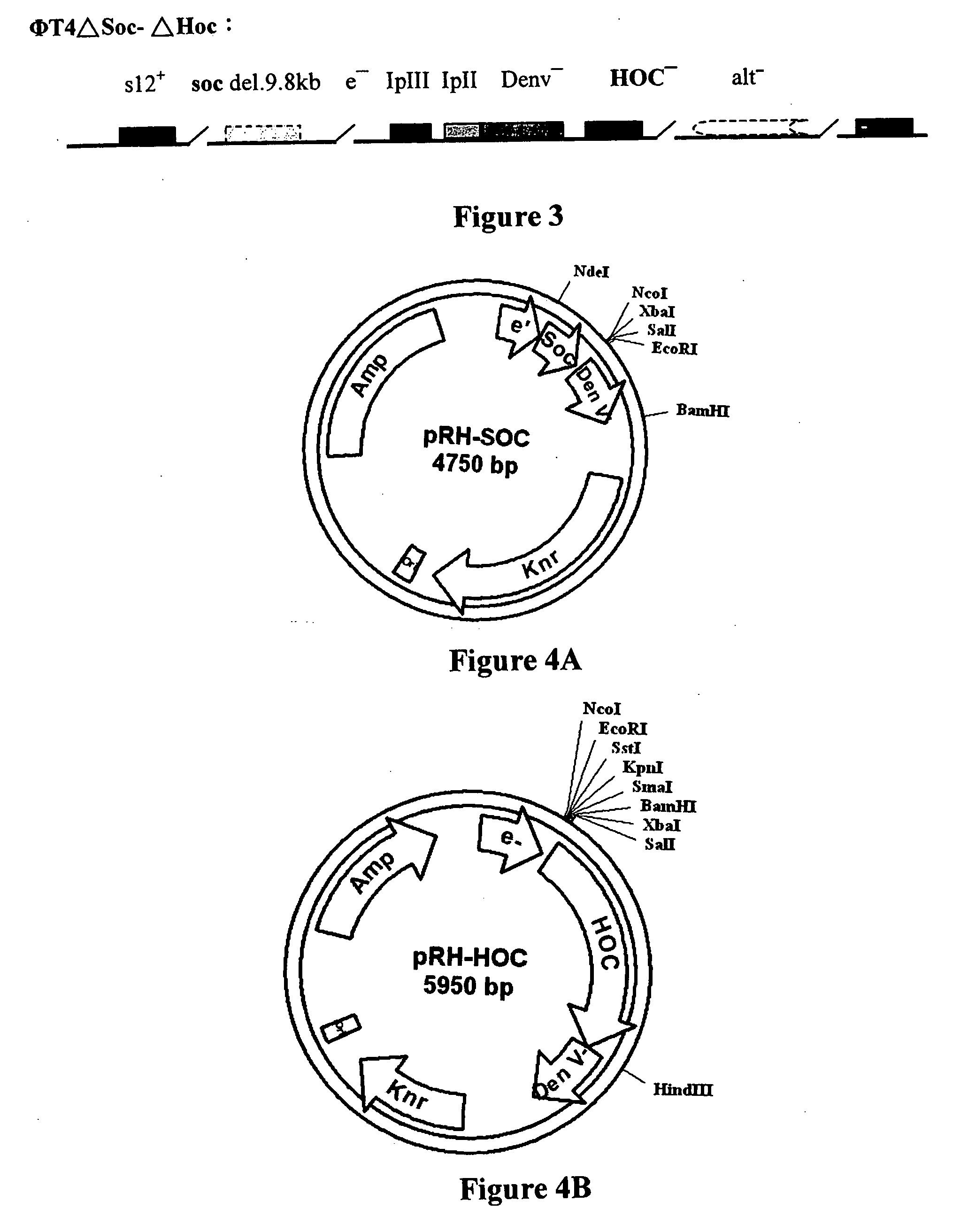
Novel recombinant T4 phage particle and uses thereof
InactiveUS20060029615A1Bacterial antigen ingredientsSsRNA viruses positive-senseBacteriophageMolecular biology
The invention is directed to a novel recombinant T4 phage particle expressing a HOC and / or SOC fusion peptide as well as methods for their preparation and methods of use in compositions and kits.
Owner:VINCOGEN CORP
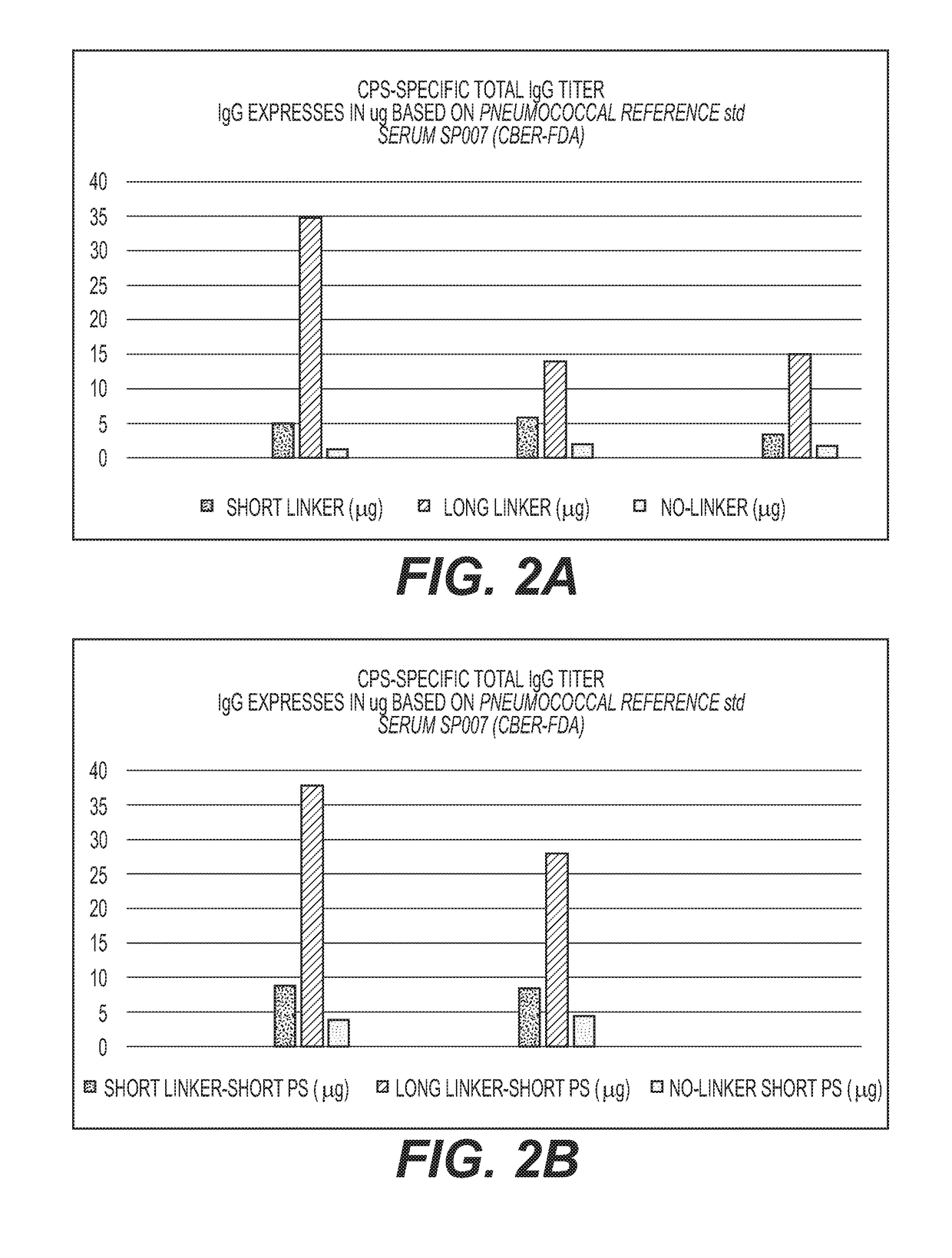
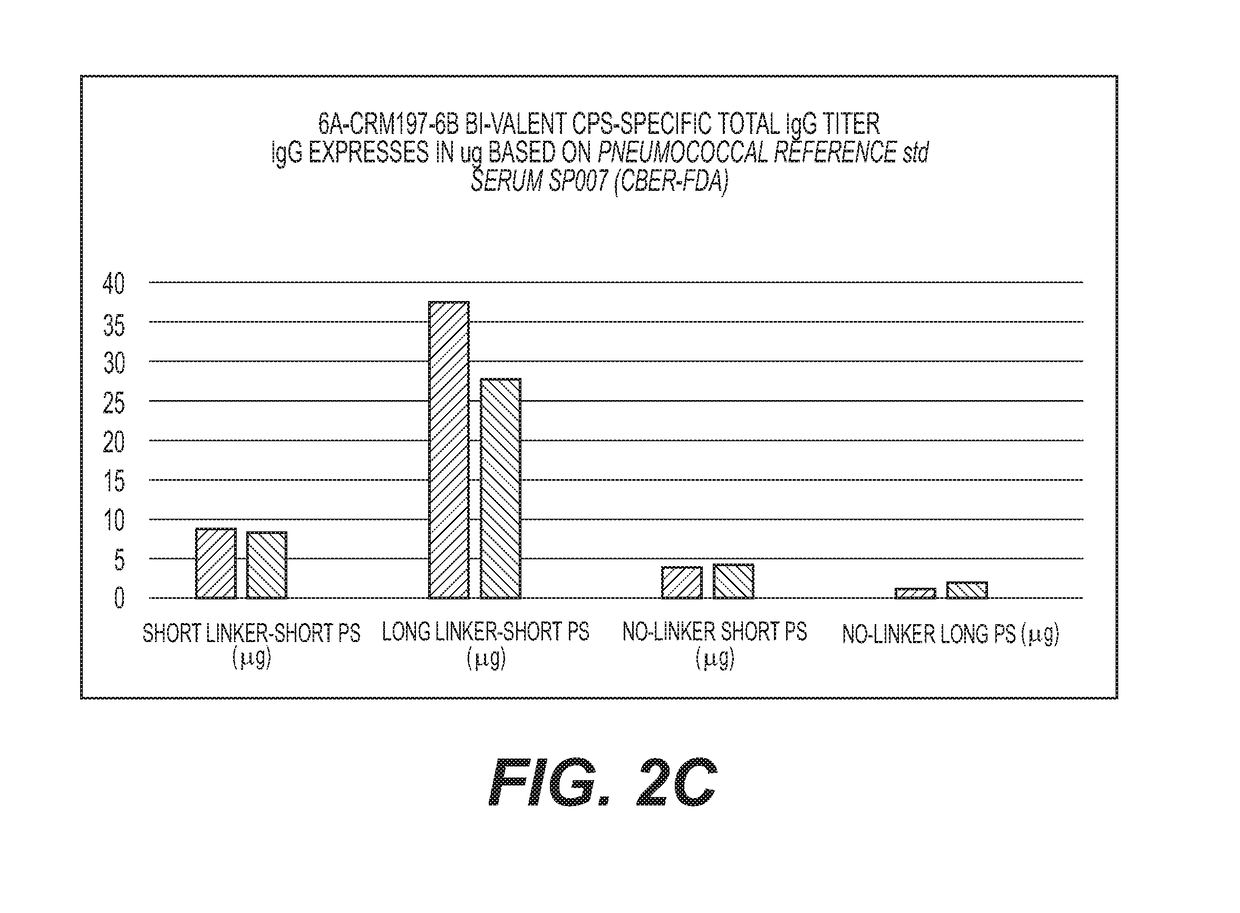
Multivalent Conjugate Vaccines with Bivalent or Multivalent Conjugate Polysaccharides that Provide Improved Immunogenicity and Avidity
ActiveUS20180353591A1Lower immune responseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineCarrier protein
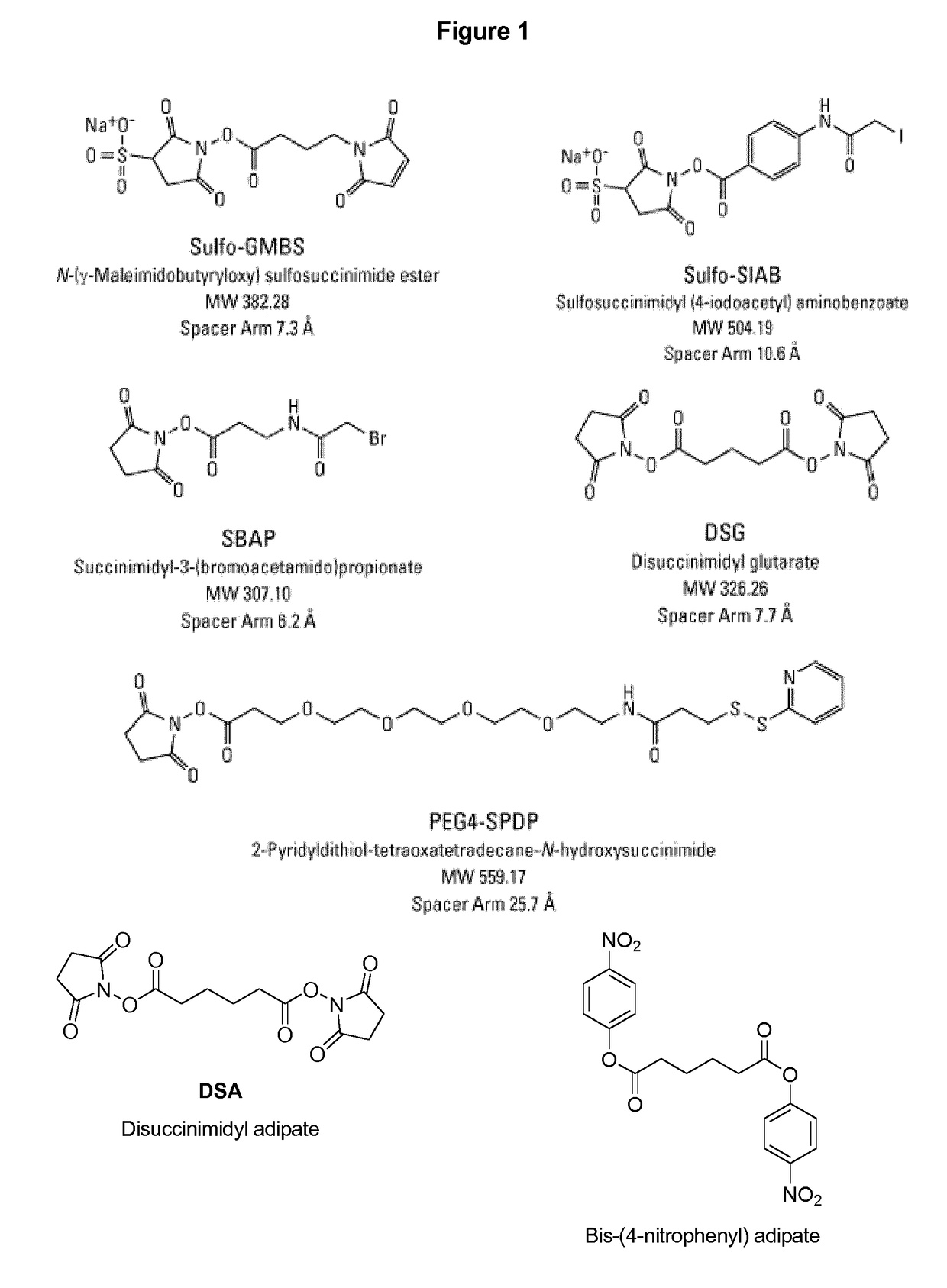
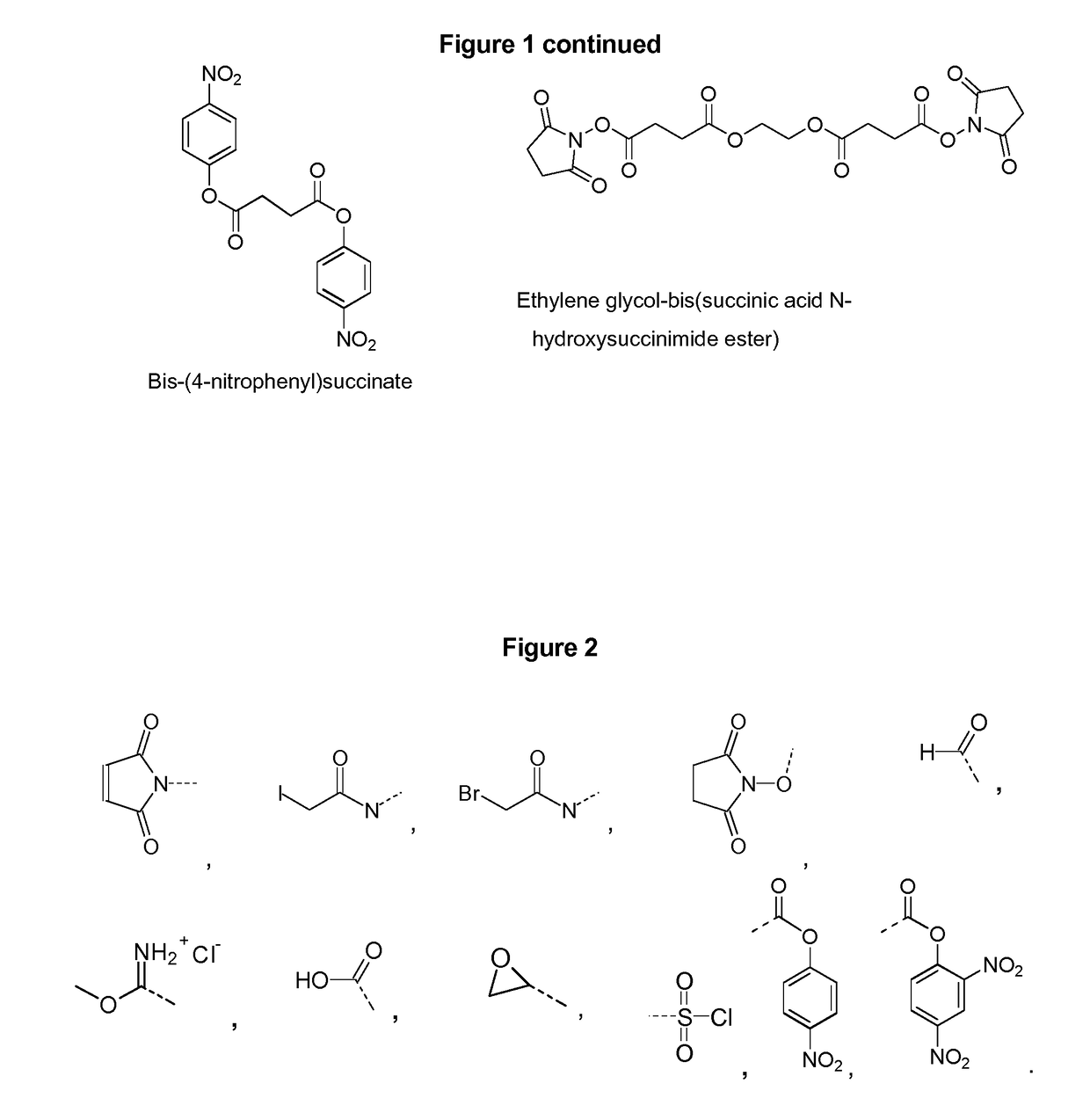
The disclosure describes compositions containing conjugates using novel linkers, bivalent polysaccharide conjugates, and methods of bivalent polysaccharide conjugation in the development of multivalent conjugate vaccines. Conjugation of capsular polysaccharides to carrier proteins is carried out using homo-bifunctional and / or hetero-bifunctional linkers of specific lengths. Incorporation of the linkers and their use in bifunctional linkers induces higher titers of functional antibodies with high avidity, eliciting higher immunologic memory, and reduced carrier protein effect. This provides immunochemically cross-reactive capsular polysaccharides wherein one or more cross-reactive capsular polysaccharides are conjugated sequentially or concurrently to carrier protein using bifunctional linkers bearing the same or different functional groups. Such a linker and the size of the capsular polysaccharides provides an effective multivalent conjugate vaccine with high antibody titers and a reduced carrier effect and results in reduction in the content of the capsular polysaccharide and protein per dose of vaccine which reduces reactogenicity.
Owner:INVENTPRISE INC
Modified Polysaccharides for Conjugate Vaccines
InactiveUS20130197203A1Good antigenicityContribute to the valency of the vaccineAntibacterial agentsDepsipeptidesCarrier proteinImmunogenicity
The present invention relates to methods of manufacture of immunogenic glycoconjugates, in particular for use in pharmaceutical compositions for inducing a therapeutic immune response in a subject. The immunogenic glycoconjugates of the invention comprise one or more oligosaccharides or polysaccharides that are conjugated to one or more carrier proteins via an active aldehyde group. Accordingly, the invention provides methods of making (i) unsaturated microbial N-acyl derivative oligosaccharides or polysaccharides; (ii) novel conjugates of unsaturated N-acyl derivatives; and (iii) glycoconjugate compositions comprising conjugate molecules of fragments of microbial unsaturated N-acyl derivatives that serve as a covalent linker to one or more proteins. The invention further encompasses the use of the immunogenic glycoconjugates pharmaceutical compositions for the prevention or treatment of an infectious disease.
Owner:PFIZER IRELAND PHARM CORP
Conjugation process of bacterial polysaccharides to carrier proteins
ActiveUS20120321660A1Size of it can retainAntibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
Process for conjugation of bacterial saccharides including Streptococcus pneumoniae and Haemophilus influenzae saccharides by reductive amination are provided herein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions, Methods, and Kits for Eliciting an Immune Response
InactiveUS20110142874A1Improving immunogenicityHigh potencySugar derivativesPeptide preparation methodsImmunogenicity
The present invention relates to compositions, methods, and kits for eliciting an immune response to at least one antigen, in particular for enhancing antigen immunogenicity.
Owner:ARIZONA STATE UNIVERSITY
Cellular immunity inducing vaccine
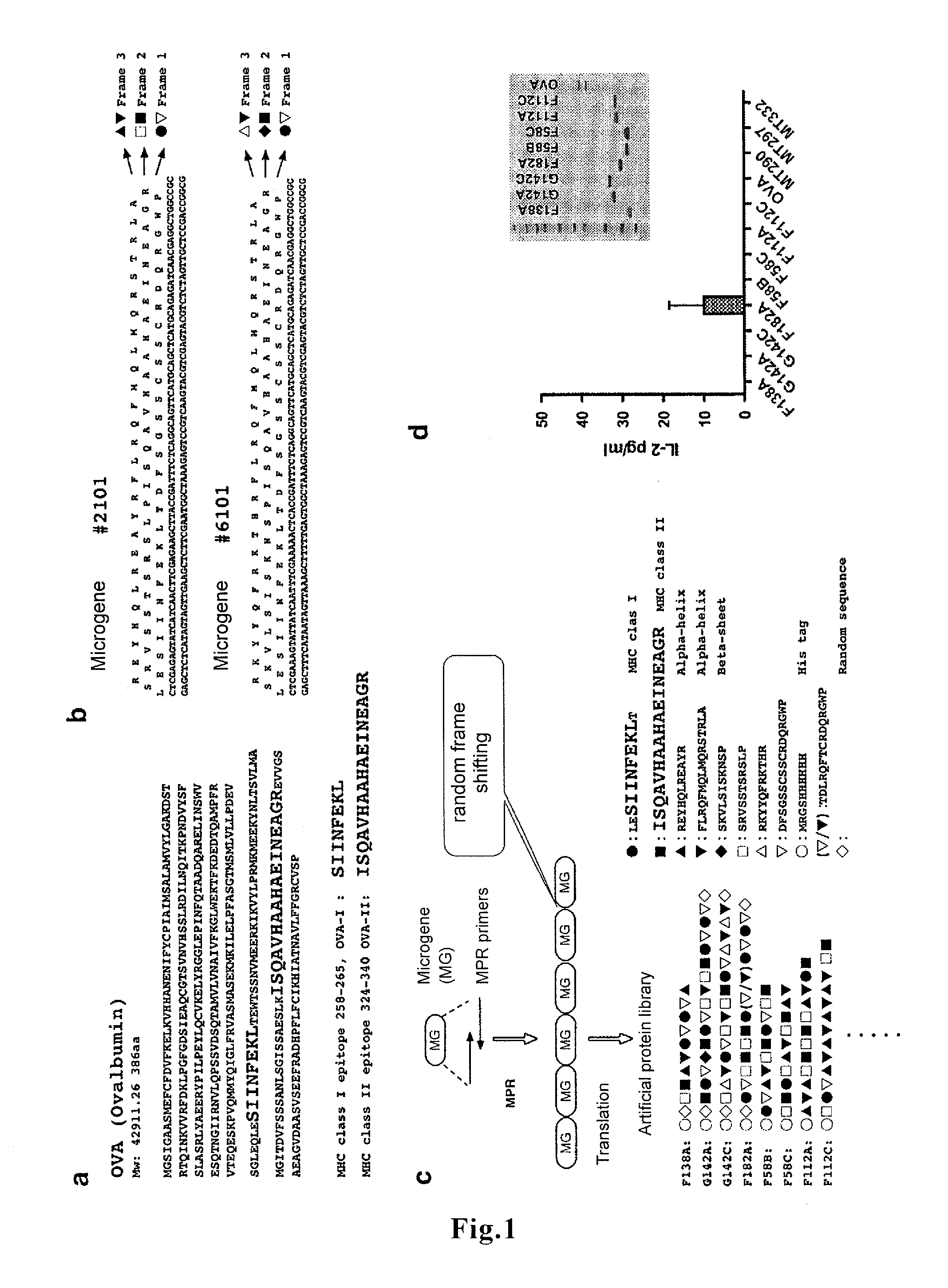
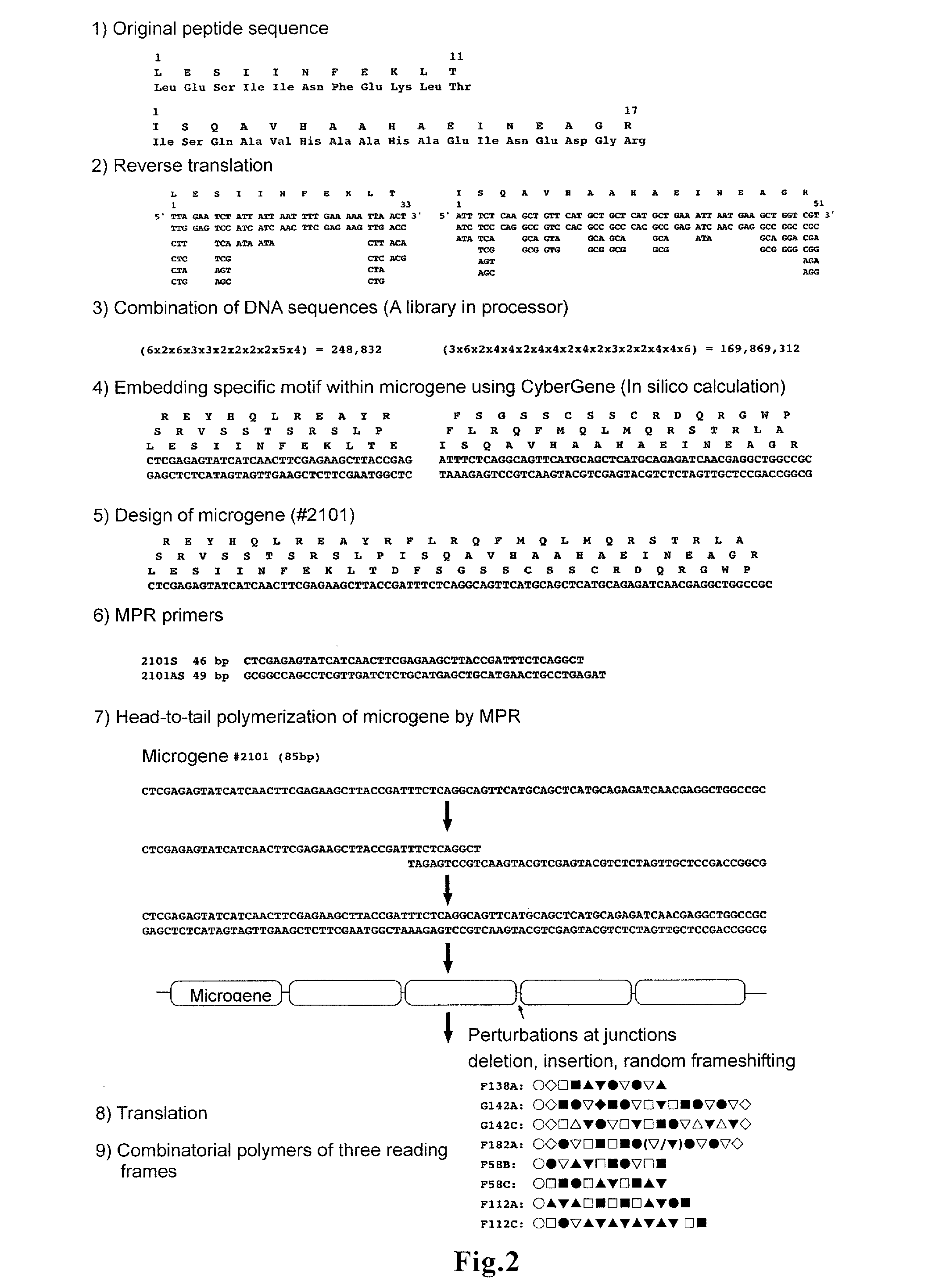
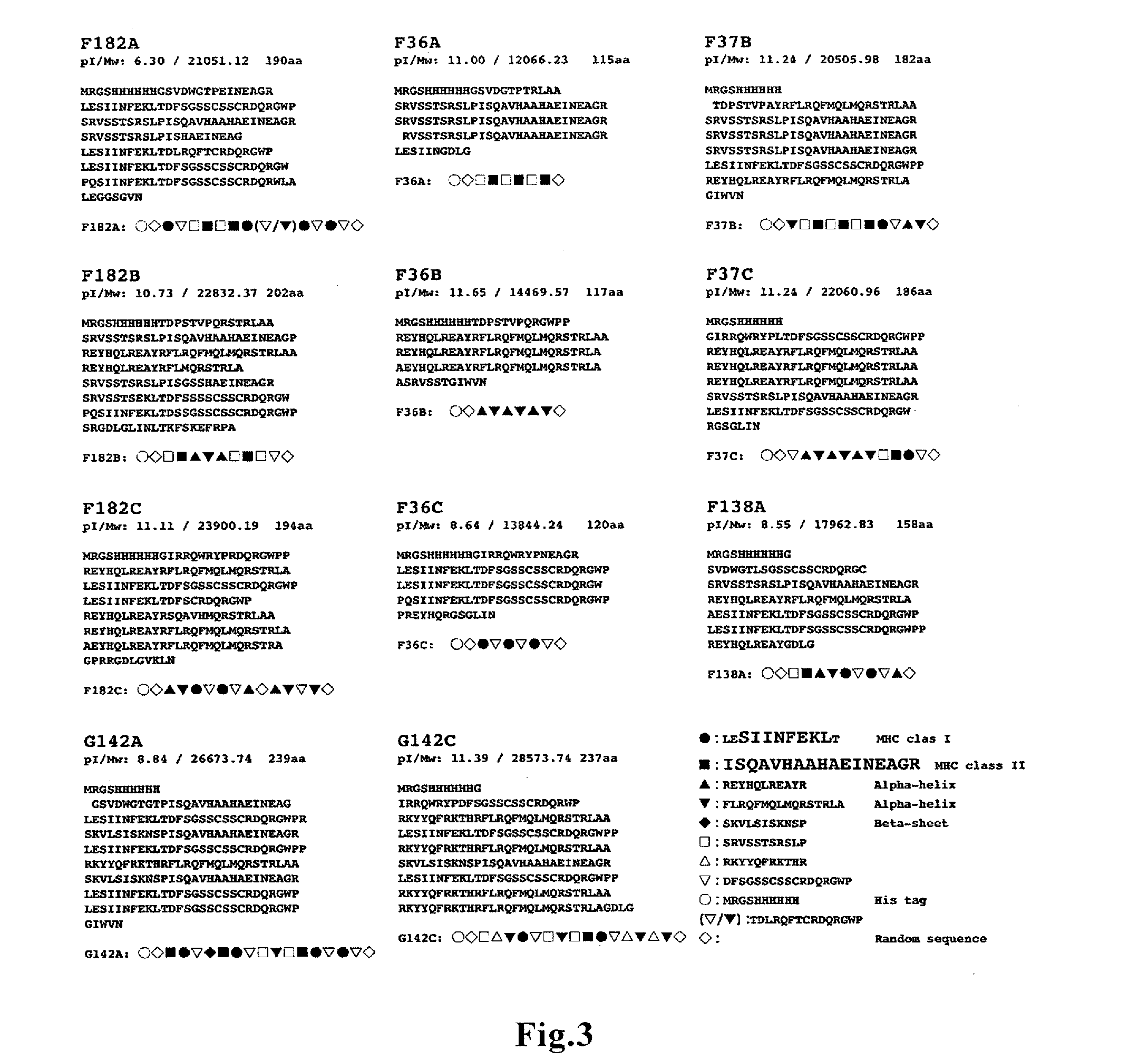
ActiveUS20160166665A1Large capacityHigh immune-inducing capacityTumor rejection antigen precursorsTumor specific antigensMHC class IHigh cell
A novel vaccine that can induce sufficiently high cell-mediated immunity is disclosed. The vaccine of the present invention contains, as an effective component, a polypeptide comprising a tandem repeat structure in which an MHC class I epitope region derived from an antigen protein and a spacer sequence are linked to each other alternately and repeatedly at least three times, or a recombinant vector which comprises a polynucleotide encoding said polypeptide and is capable of expressing said polypeptide in vivo. The spacer sequence is, for example, a sequence generated as an amino acid sequence inevitably encoded by a single base sequence which is designed such that the MHC class I epitope region derived from the antigen protein, an MHC class II epitope region derived from the antigen protein, and at least one higher-order-structure-stabilizing region are encoded by different reading frames in said single base sequence.
Owner:JAPANESE FOUND FOR CANCER RES +1
Carrier of a diamond fine particle for immobilizing virus
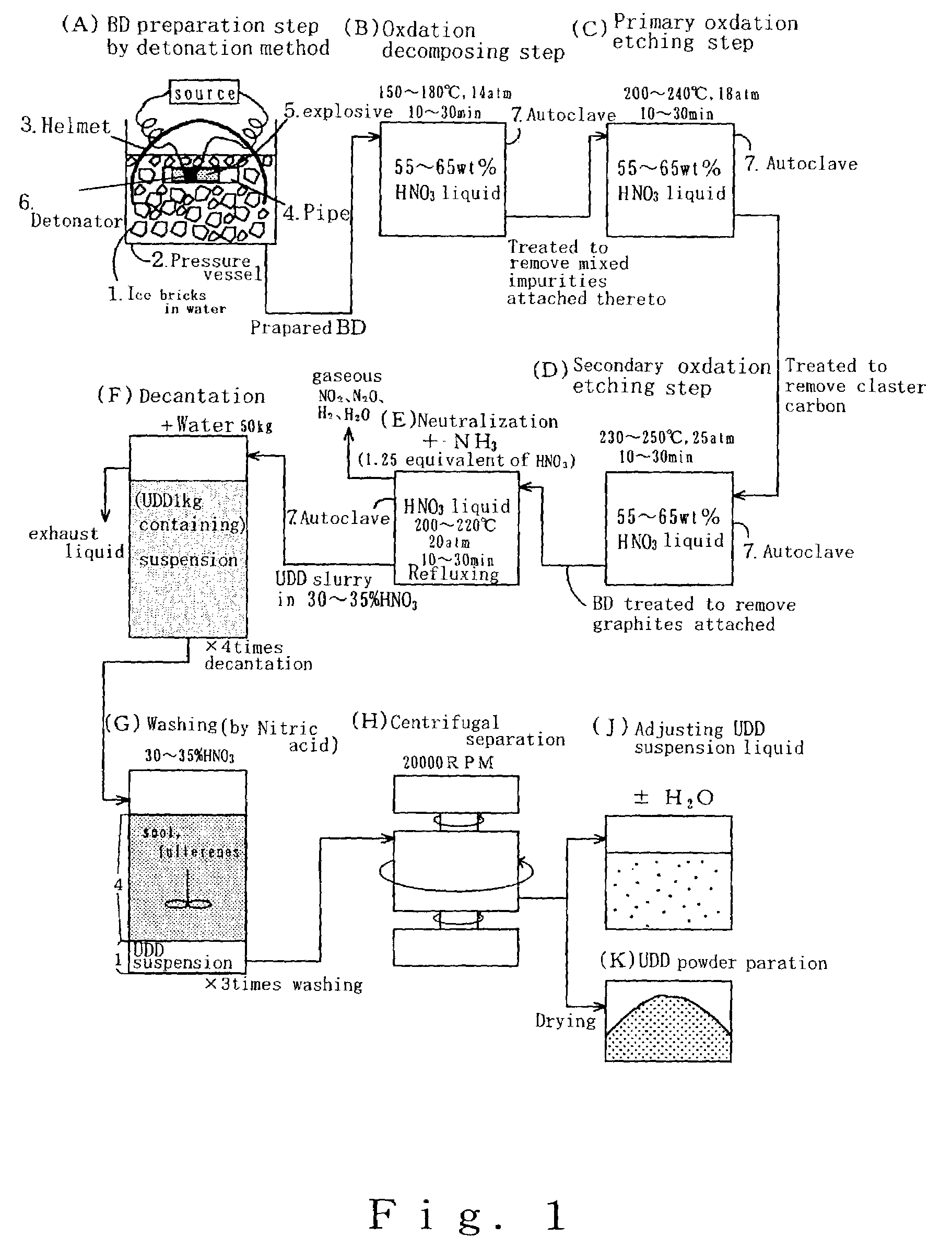
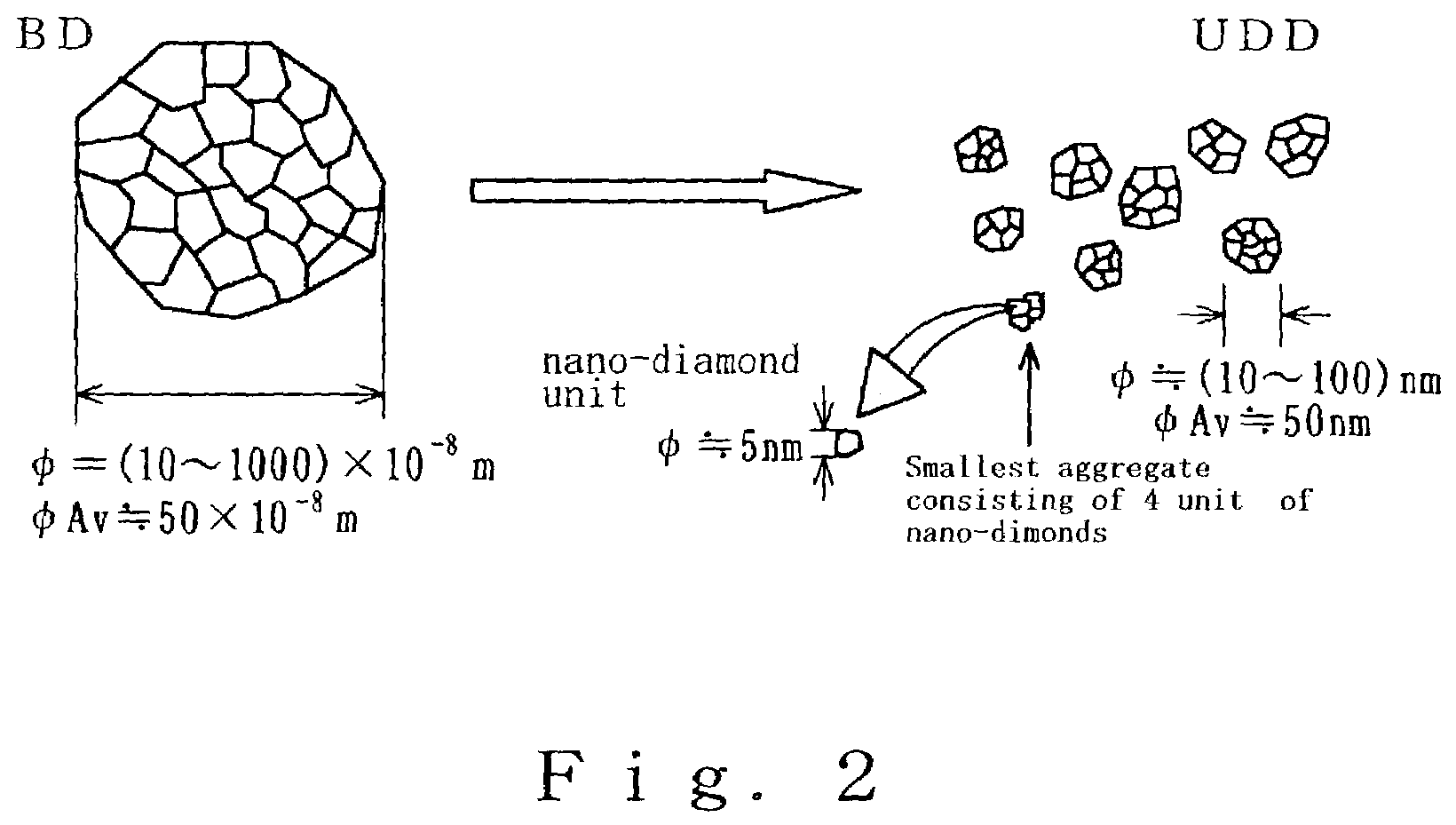
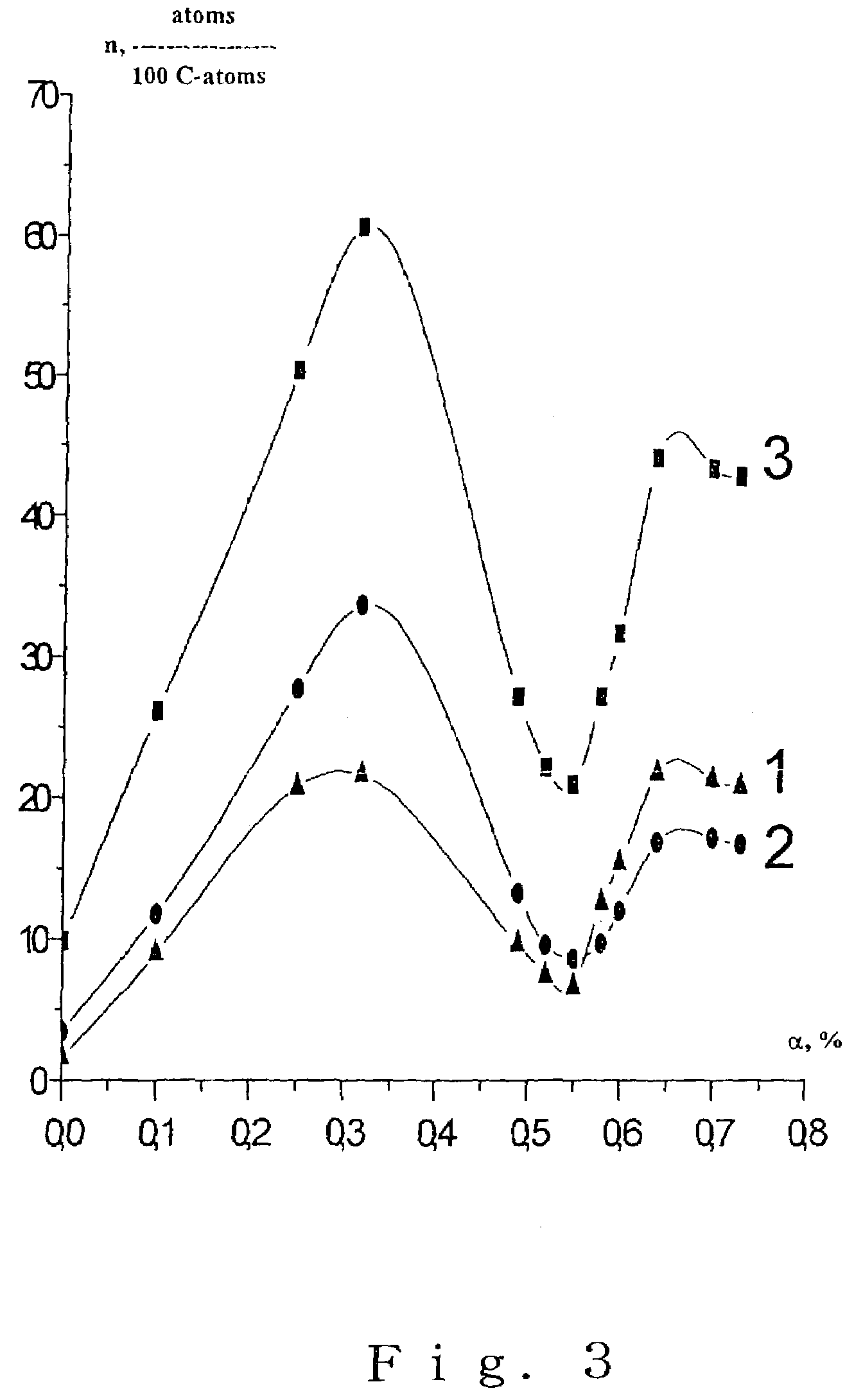
ActiveUS7491554B2Improve hydrophilicityGood biocompatibilityMaterial nanotechnologyPowder deliveryVirusPolymer
The present invention provides a carrier for immobilizing a bio-derived polymer, and especially, the present invention provides a carrier of diamond fine particle for immobilizing a DNA, RNA, protein and peptide, as well as a DNA chip substrate, a carrier for trapping virus and a vaccine. The carrier is prepared by the steps comprising: preparing an initial mixture including a diamond and a non-diamond by means of explosion of a detonating agent; subjecting the initial mixture to an oxidation treatment to obtain a suspension solution including the diamond; and separating a phase including the diamond. According to the present invention, after the oxidation treatment, a basic material having per se volatility or to generate a decomposed material having volatility is added, to neutralize with nitric acid, and thereby obtained diamond fine particle has a functional group on the surface thereof.
Owner:NICCA CHEM COMPANY
Process for preparing polysaccharide-protein conjugate vaccines
ActiveUS9173931B2High yieldReduce productionAntibacterial agentsBacterial antigen ingredientsConjugate vaccineHydrazide
Methods for the manufacture of polysaccharide-protein conjugate vaccines at high yield are provided. The methods involve reaction of a hydrazide group on one reactant with an aldehyde group on the other reactant. The reaction proceeds rapidly with a high conjugation efficiency. Simplified purification processes can be employed to separate the conjugate product from the unconjugated protein and polysaccharide and other small molecule by-products.
Owner:DEPT OF HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC THE +1
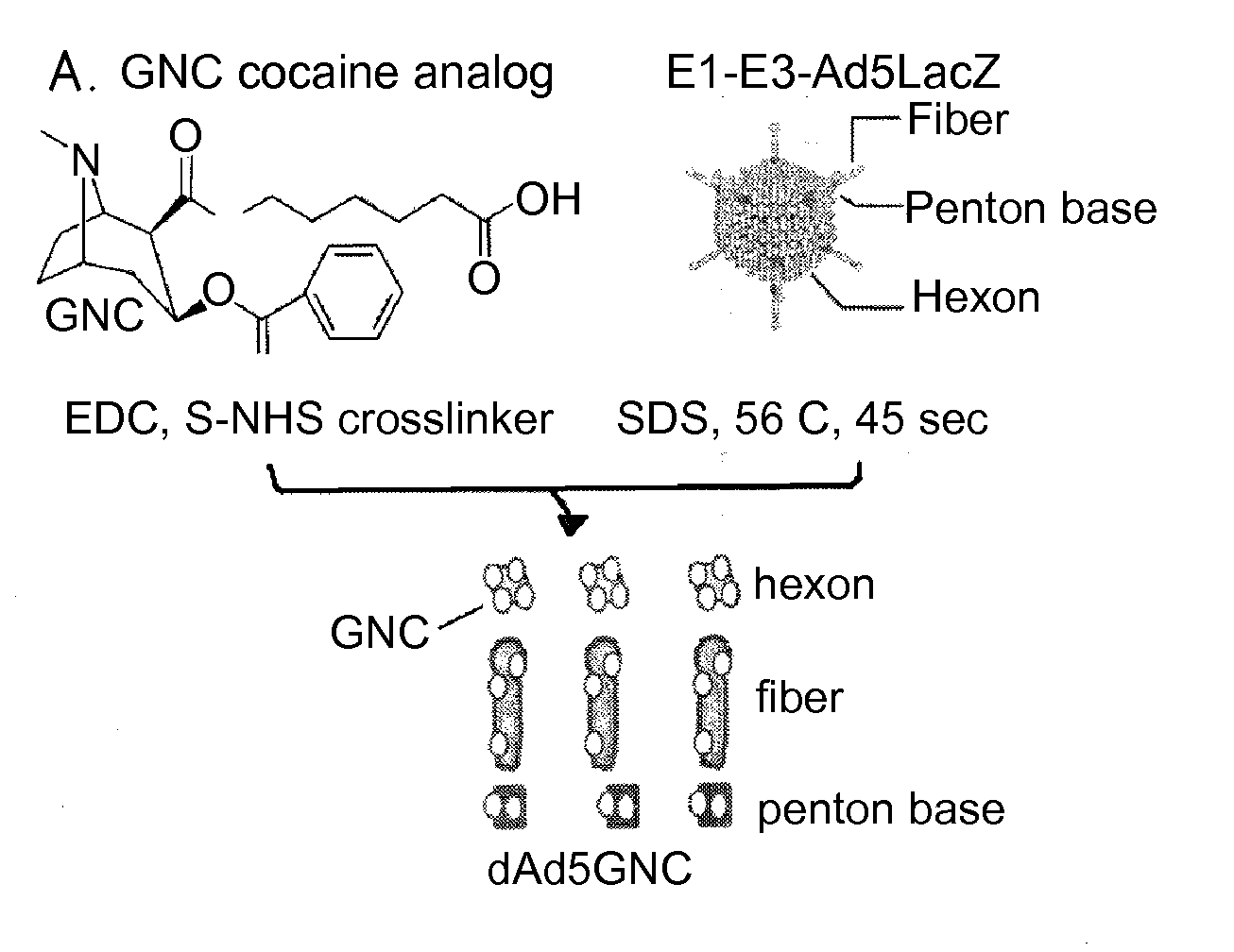
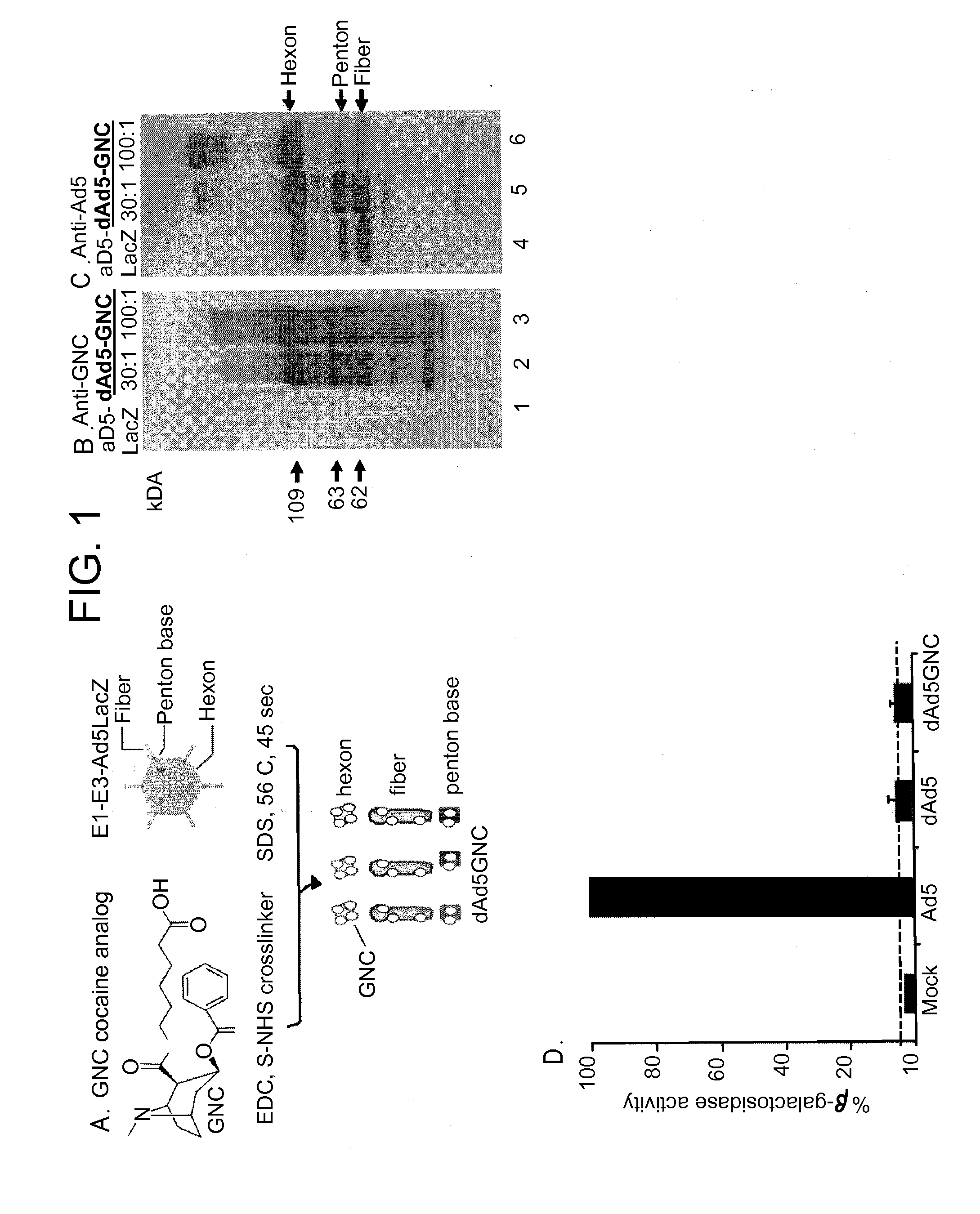
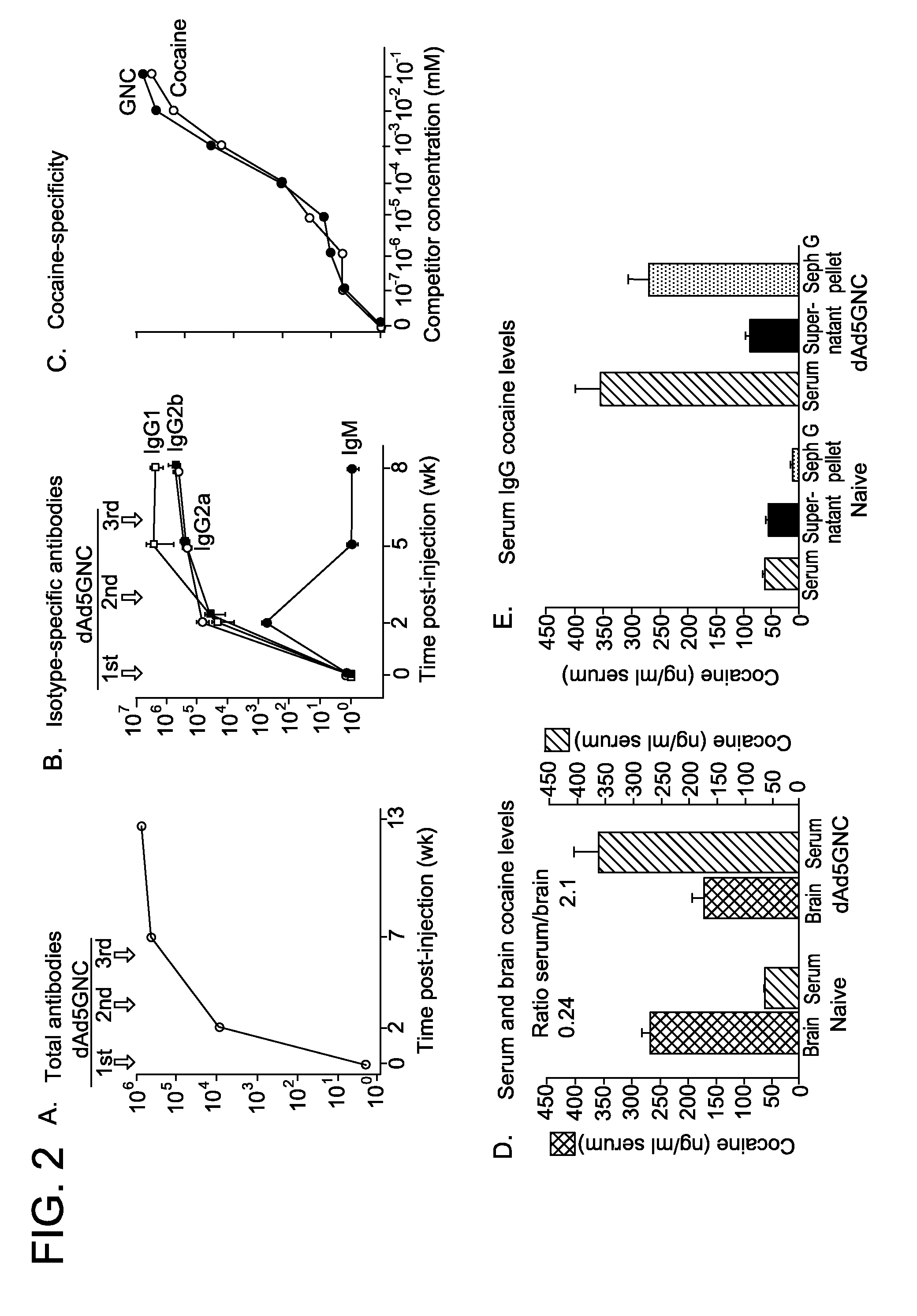
Disrupted adenovirus-based vaccine against drugs of abuse
The invention is directed to an adenovirus-antigen conjugate comprising (a) a disrupted adenovirus with a coat protein and (b) an antigen conjugated to the coat protein of the disrupted adenovirus, as well as a conjugate comprising (a) a disrupted adenovirus with a coat protein and (b) an antigen conjugated to the coat protein of the disrupted adenovirus. The invention also provides a method of inducing an immune response against an antigen in a human using the aforementioned conjugates. The invention further provides an adeno-associated viral vector comprising a nucleic acid sequence which encodes an antibody directed against cocaine.
Owner:CORNELL UNIVERSITY
Complete virus gene engineering vaccine of aftosa and its preparation method
InactiveCN1602962ANot contagiousLow costSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleic Acid ProbesViral Vaccine
The invention discloses a kind of foot-and-mouth disease genetic engineering vaccine and its the preparing method.Its structure expression is: Entire virus genetic engineering vaccine: Phage T4-Soc-Hoc-FMDV-P1; Asian unit genetic engineering vaccine: Phage T4-Soc-Hoc-FMDV-F3.The preparing method is: Takes O blood serum foot-and-mouth disease virus poisonous, extracts total gene group RNA; From blood serum poisonous DNA measure foreword to synthesizes two DNA nucleic acid probe;takes the probe as the directing thing, carries on Rt-pcr chain type DNA expand increase response, obtains the viral granule P1gene;gene recombinate the P1gene and the T4bacteriophage material particle, pRH-Soc, obtains T4bacteriophage conformity material particle pRH-Soc-P1;homology reorganizes pRH-Soc-P1with the T4bacteriophage carrier phage T4- í¸ Soc & í¸ Hoc, then enters in the backwoods coli E.coli.CR63host mycelium, obtains this invention genetic engineering vaccine phage T4-Soc-FMDV-P1.compares this invention with the deactivation entire viral vaccine, it does not have to fight fire, maintains the entire viral antigenicity but not have the infection.may inject, also may take orally, convenient, quick, the immunity effect is good.
Owner:任兆钧
Vaccines against Streptococcus pneumoniae serotype 8
ActiveUS10220083B2Improving immunogenicityImprove flow characteristicsAntibacterial agentsOrganic active ingredientsProtection sexAntibody
The present invention relates to synthetic saccharides of general formula (I) that are related to Streptococcus pneumoniae serotype 8 capsular polysaccharide, conjugates thereof and the use of said saccharides and conjugates for raising a protective immune response in a human and / or animal host. Furthermore, the synthetic saccharide structures of general formula (I) are useful as marker in immunological assays for detection of antibodies against Streptococcus pneumoniae bacteria.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
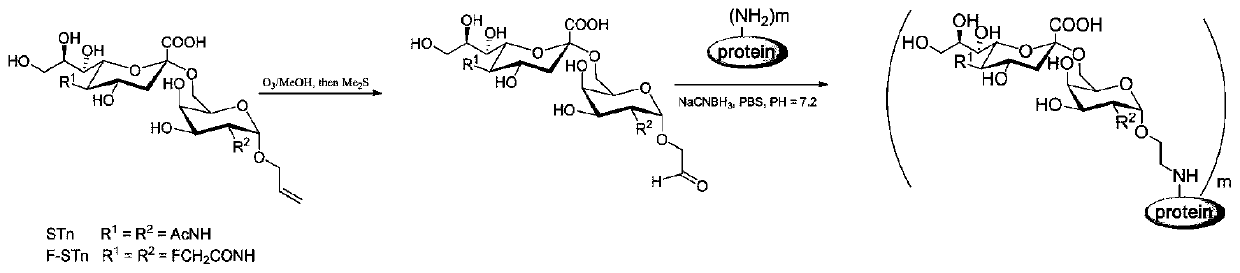
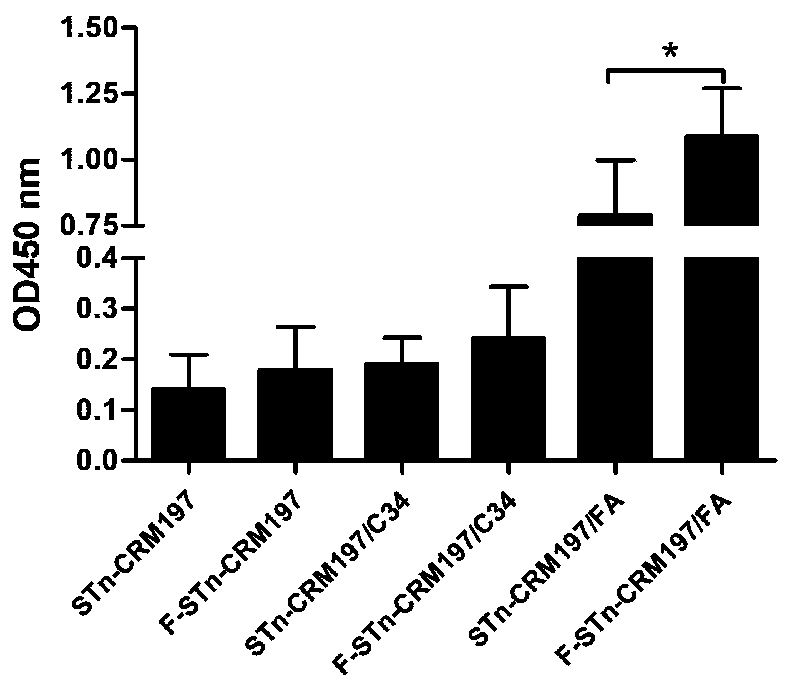
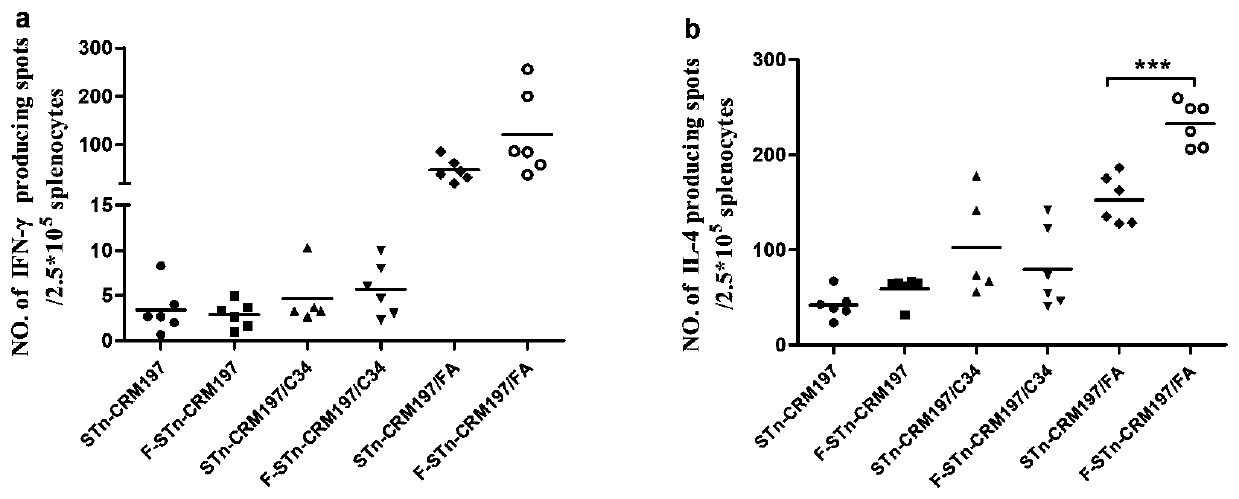
Glycoconjugate containing STn or F-STn and preparation method thereof and application for anti-tumor vaccine
ActiveCN110064050AEnhance immune responseEfficient identificationCarrier-bound antigen/hapten ingredientsPharmaceutical non-active ingredientsGlycoconjugateHumoral immune reaction
The invention provides a glycoconjugate containing STn or F-STn and a preparation method thereof and application for an anti-tumor vaccine, and belongs to the technical field of glycoconjugates. The glycoconjugate containing STn or F-STn comprises STn or F-STn and a vector; STn or F-STn is coupled to the vector through a connecting bond; the connecting bond includes an amido bond or an oxime bondor a C-N bond; the vector includes protein or zwitter-ion polysaccharide or lipid or nucleic acid. The glycoconjugate is applied to preparing an anti-tumor or anti-cancer vaccine or preparing a medicine for improving cell and / or humoral immune response. The vaccine prepared from the glycoconjugate of F-STn can improve humoral immunity and cellular immunologic response, the generated antibody can effectively recognize STn positive tumor cells, and the STn positive tumor cells can be cracked in an ADCC and CDC way.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com