Group A streptococcus oligosaccharide protein conjugate as well as preparation method and application thereof
A group A streptococcus and protein conjugate technology, applied in the direction of antibacterial drugs, pharmaceutical formulations, bacterial antigen components, etc., to achieve effective inhibition and good applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
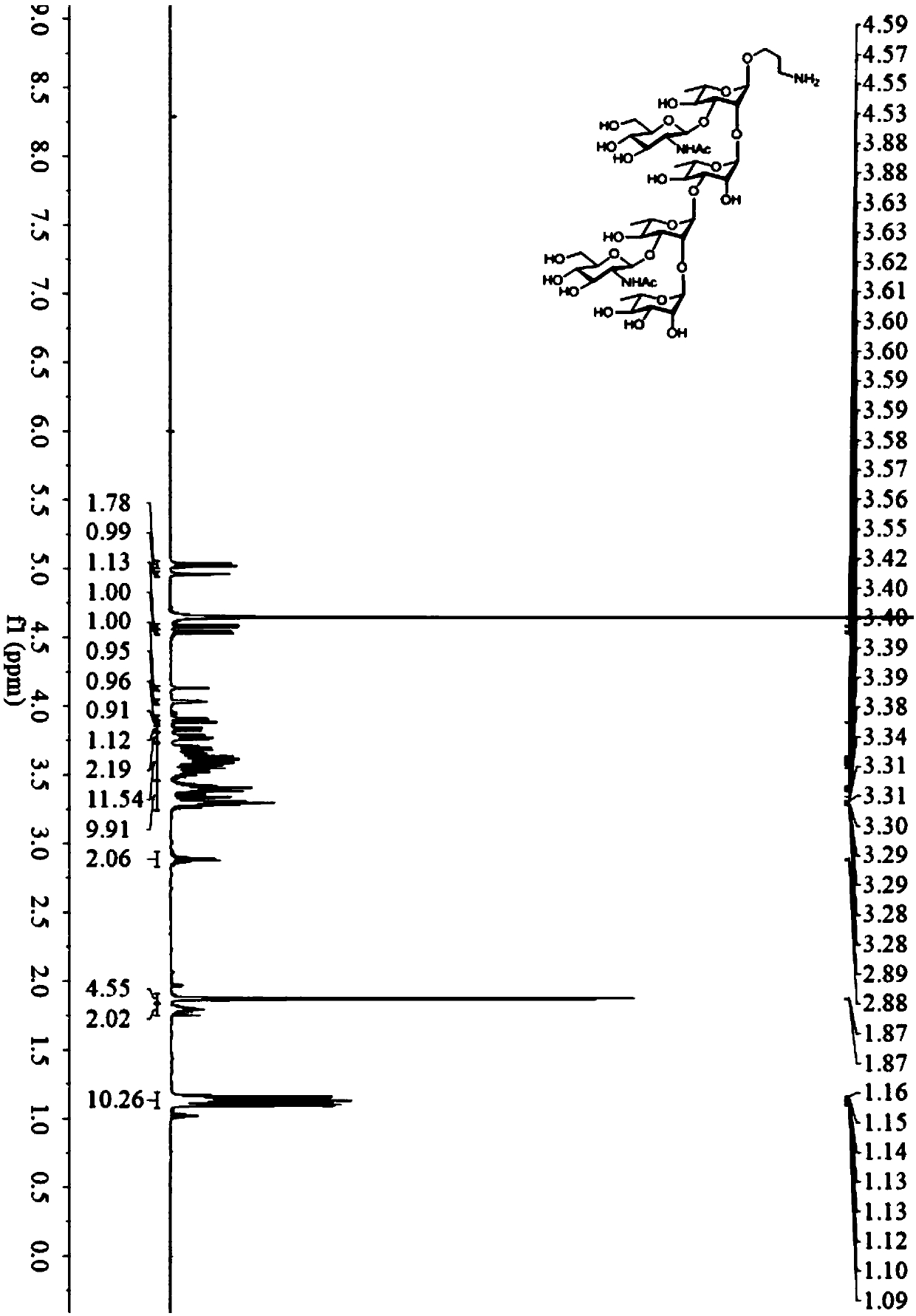
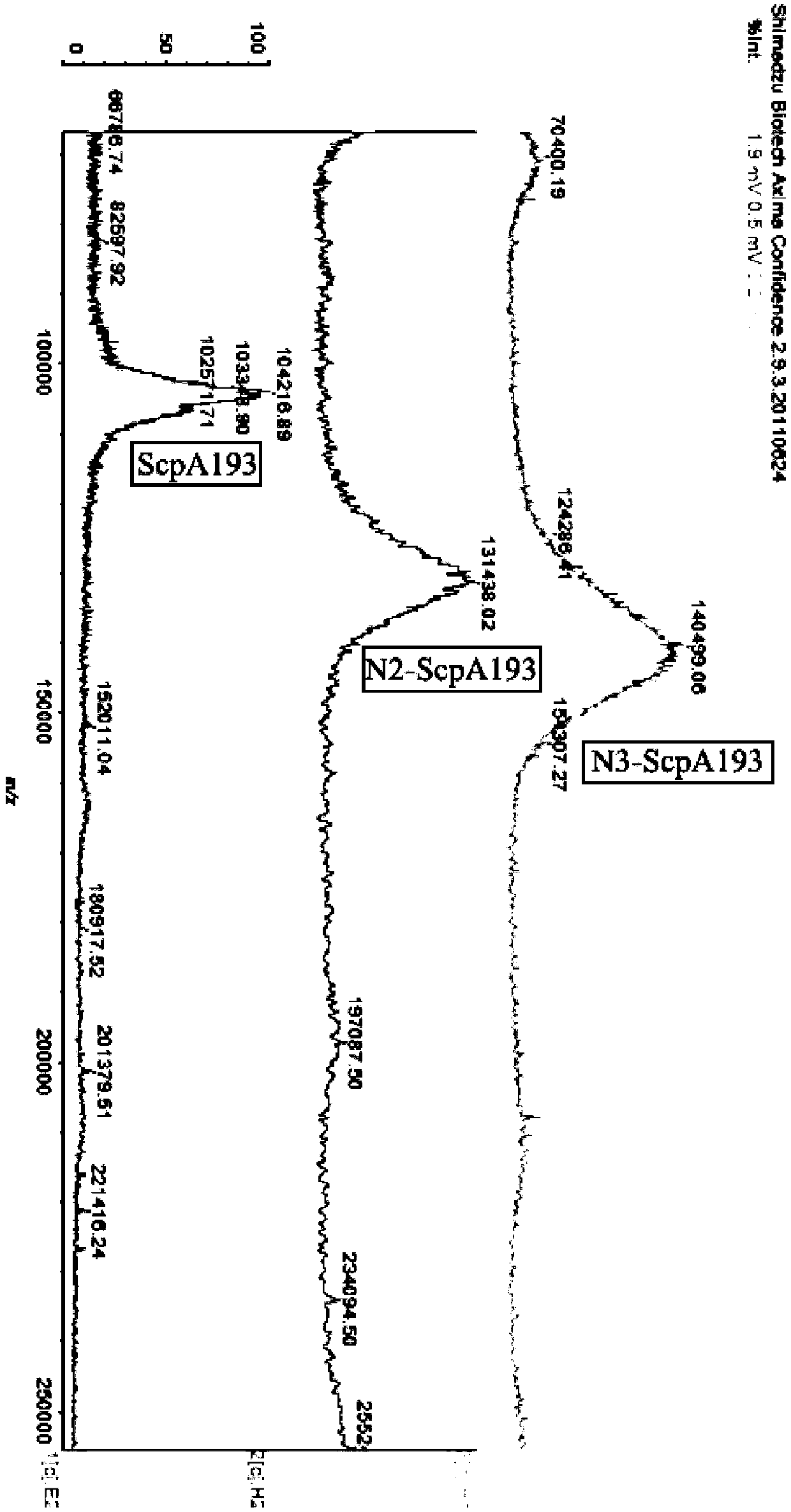
[0042] Example 1: 3-aminopropyl α-L-rhamnopyranosyl-(1→2)-[2-deoxy-2-acetylamino-β-D-glucopyranosyl-(1→3) ]-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→2)-[2-deoxy-2-acetylamino-β-D- Synthesis of Glucopyranosyl-(1→3)]-α-L-rhamnopyranose-ScpA193 Conjugate (N2-ScpA193)
[0043] 1. Synthesis of GAS oligosaccharide antigen N2
[0044] 1) Synthesis of Compound H1
[0045]
[0046] Under the protection of nitrogen, the compound 2-deoxy-2-phthalamido-3,4,6-triacetylglucopyranosamine trichloroacetimidate (2.52g, 4.35mmol) (Journal of Organic Chemistry 2005,70(1),214-226), 4-oxo-benzoylrhamnopyranosopropylglucoside (1.26g,4.14mmol)(CarbohydrateResearch.2011,346(17),2801-2804) 15mL of dry dichloromethane was dissolved and transferred to the activated In the molecular sieve reaction flask, add dry dichloromethane to 50 mL, stir at room temperature, after 30 minutes, add ice-water bath to cool down to 0°C, and add trimethylsilyl trifluoromethanesulfonate (79 μL, 0.435 mmol) d...
Embodiment 2
[0082] GAS oligosaccharide antigen N2 was synthesized according to the method described in Example 1.
[0083] A preparation method of group A streptococcal oligosaccharide protein conjugate N2-ScpA193, the steps are as follows:
[0084] (1) Activation of N2: Dissolve the GAS oligosaccharide antigen N2 (2 mg) prepared above in 1 mL of N,N-dimethylformamide (DMF) and phosphate buffered saline (PBS buffer, Na 2 HPO 4 0.1M, KH 2 PO 4 0.1M, pH 8) mixed solution (DMF:PBS=4:1, volume ratio), add disuccinimide glutarate to it under stirring condition, disuccinimide glutarate and GAS oligo The molar equivalent ratio of carbohydrate antigen N2 was 10:1, stirred at room temperature for 4 hours, and the solvent was distilled off under reduced pressure to obtain a white powdery solid, which was washed with EA (ethyl acetate) and dried to obtain activated GAS oligosaccharide antigen N2;
[0085] (2) Synthesis of the conjugate: Dissolve the activated GAS oligosaccharide antigen N2 and...
Embodiment 3
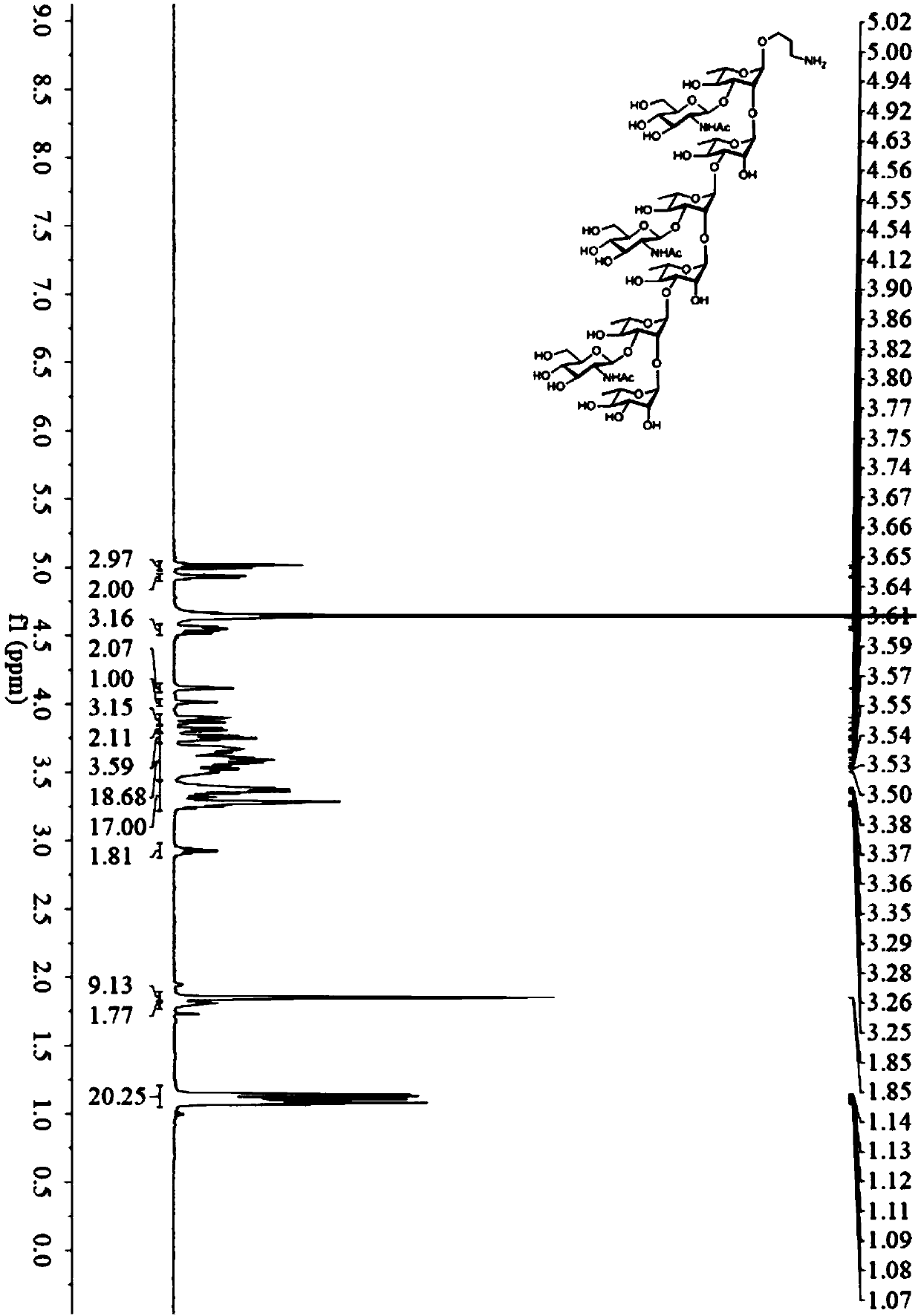
[0089] Example 3: 3-aminopropyl α-L-rhamnopyranosyl-(1→2)-[2-deoxy-2-acetylamino-β-D-glucopyranosyl-(1→3) ]-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→2)-[2-deoxy-2-acetylamino-β-D- Glucopyranosyl-(1→3)]-α-L-rhamnopyranosyl-(1→3)-α-L-rhamnopyranosyl-(1→2)-[2-deoxy -Synthesis of 2-acetylamino-β-D-glucopyranosyl-(1→3)]-α-L-rhamnopyranose-ScpA193 conjugate (N3-ScpA193)
[0090] 1. Synthesis of GAS oligosaccharide antigen N3
[0091] 1) Synthesis of Compound H10
[0092]
[0093] Under nitrogen protection, compound H4 (97mg, 0.126mmol) and compound H6 (161mg, 0.137mmol) prepared in Example 1 were transferred to an activated In a molecular sieve reaction vial, the solution was stirred at room temperature for 30 min. Then the temperature of the mixed solution was lowered to 0° C., and iodosuccinimide (42 mg, 0.187 mmol) and trimethylsilyl trifluoromethanesulfonate (3.4 μL, 18.7 μmol) were added. The reaction solution was stirred at 0°C for 1 hour and then slowly warme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com