Cell-free preparations of immunopotentiators, and preparation methods and uses thereof
A technology of cell-free preparation and Pseudomonas preparation is applied in the field of cell-free preparations of various immune enhancers, which can solve the problems of cell fragmentation and difficulty in realization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
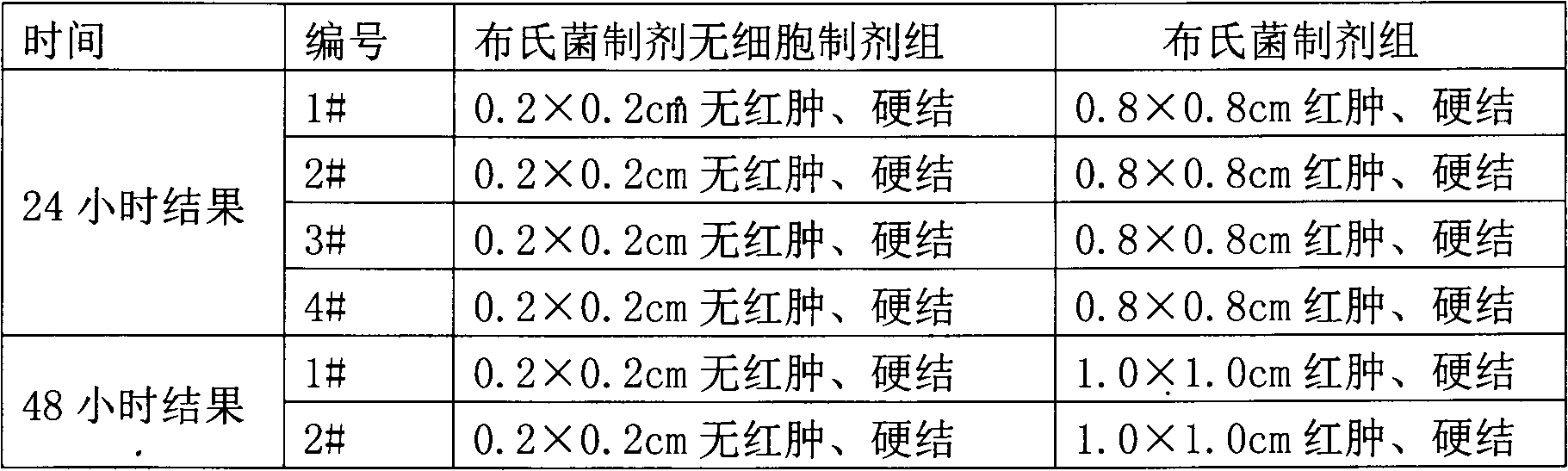
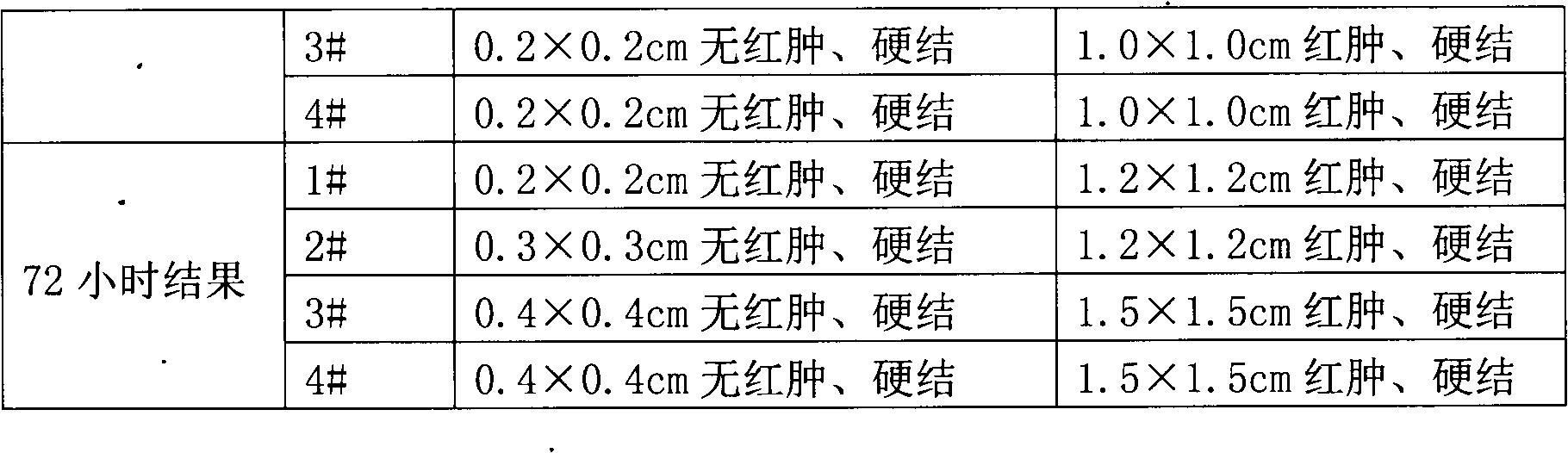
[0067] Embodiment 1 treatment uses the preparation of Brucella preparation cell-free preparation
[0068] 1. Preparation of Brucella preparations for treatment
[0069] According to the method described in the 2000 edition of "China Biological Products Regulations", the establishment, verification and other verification of bacterial seed batches were carried out.
[0070] 1. Seeds for production: After opening the batch of working seed strains, inoculate them on the slant of liver infusion agar or other suitable medium, and cultivate them at 37°C for 44-48 hours to form the first-generation strains. The first-generation strains can be stored at 2-8°C for 15 days; transplant the first-generation strains onto liver agar or other suitable medium, and culture them at 37°C for 44-48 hours to become the second-generation strains. After passing the pure bacteria inspection, wash the bacterial lawn with sterile physiological sodium chloride solution to make a suspension. Use this as...
Embodiment 2
[0079] The preparation of the cell-free preparation of embodiment 2 BCG polysaccharide, nucleic acid preparation
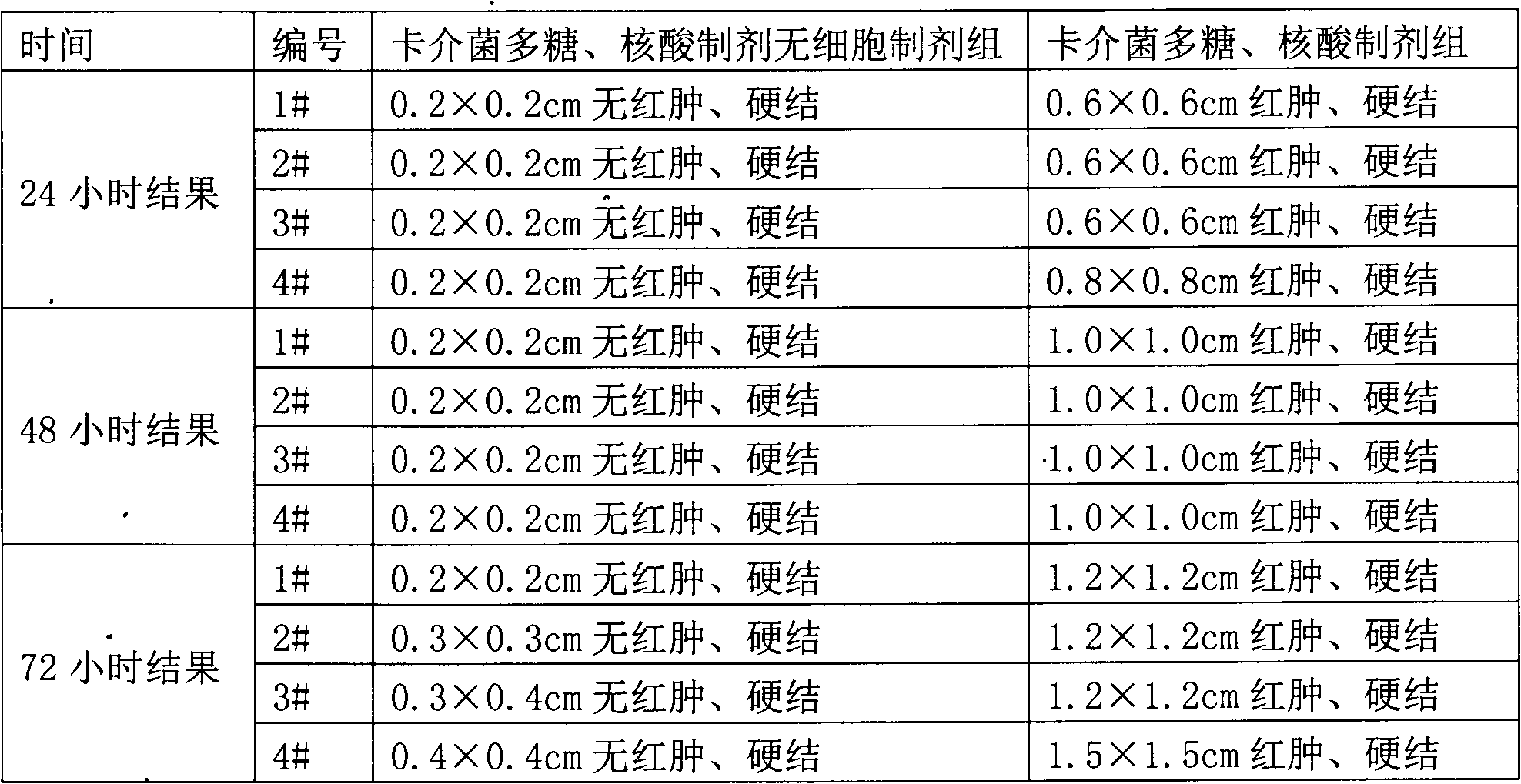
[0080] BCG polysaccharides and nucleic acid preparations are made by extracting polysaccharides and nucleic acids from BCG by thermal phenol method, and mixing them with sterilized physiological sodium chloride solution. They are used for the prevention and treatment of chronic bronchitis, colds, asthma and other diseases.
[0081] According to the method described in the 2000 edition of "China Biological Products Regulations", the establishment, verification and other verification of bacterial seed batches were carried out.
[0082] 1. Preparation of BCG polysaccharide and nucleic acid preparations
[0083] Source: The strains approved by the National Drug Administration are used for production, and the use of strains passed through animals is strictly prohibited for production.
[0084]Freeze-dried strains are passed once on Sutong potato medium, bile potato me...
Embodiment 3
[0093] Example 3: Preparation of Cell Free Preparation of Group A Streptococcus Preparation
[0094] Group A Streptococcus preparation is a freeze-dried product made from the attenuated strain of Group A Streptococcus after cultivation, potentiation and penicillin treatment, and is used for immunotherapy of cancerous pleural effusion and malignant tumors.
[0095] According to the method described in the 2000 edition of "China Biological Products Regulations", the establishment, verification and other verification of bacterial seed batches were carried out.
[0096] One, the preparation of group A streptococcus preparation
[0097] The strain is CMCC32235 strain or SIPI722 strain, which is inoculated in Todd-Hewitt broth or yeast extract or other suitable solid or liquid medium.
[0098] After opening the batch of working seed bacteria, inoculate it on a suitable solid or liquid medium, cultivate it at 36±1°C, and amplify it for 2 to 4 generations, so as to prepare a suitable...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com