Therapeutic vaccine for malignant tumors and composition thereof
A technology for therapeutic vaccines and malignant tumors, applied in the field of therapeutic vaccines for malignant tumors and their compositions, and can solve problems such as cell fragmentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation method of lung cancer vaccine:
[0034] 1. Preparation of human TGF-β2 antisense plasmid:
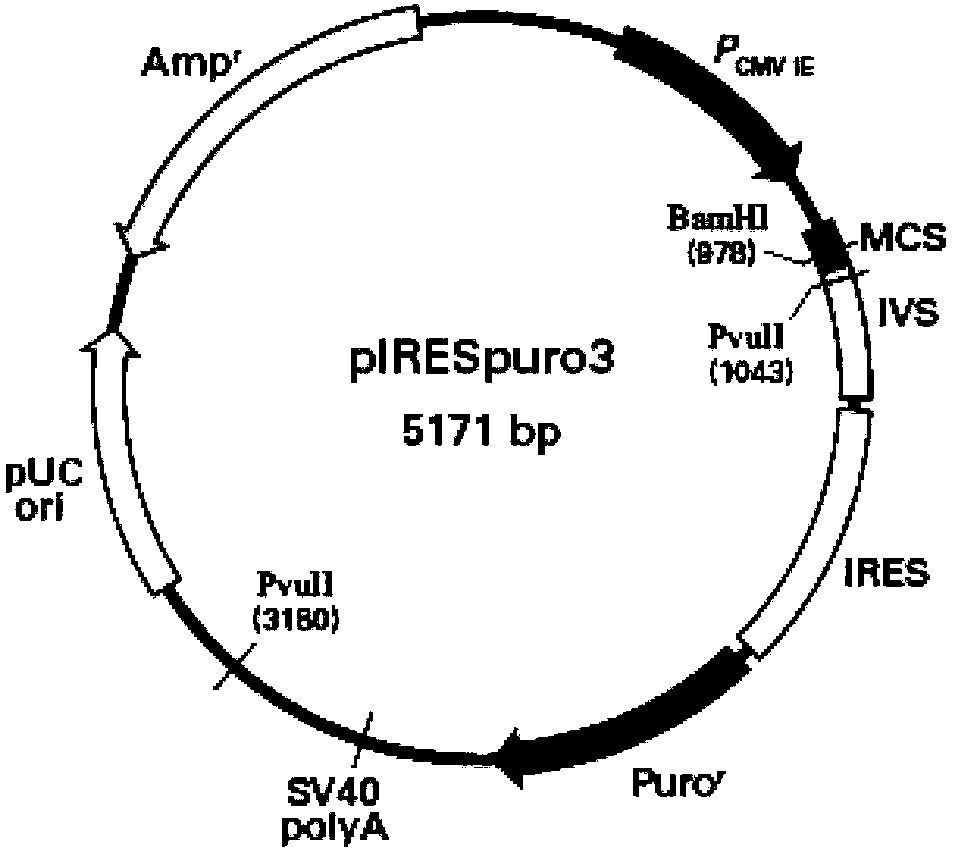
[0035] The cDNA of human TGF-β2 (NCBI Reference Sequence: NM_003238.3, fragment 1369bp-2613bp) was cloned in the reverse direction into the multiple cloning site MCS of pIRESpuro3 vector, which was confirmed by sequencing. Because the pIRESpuro3 vector contains the promoter and enhancer of CMV, it can replicate in mammals; it contains the internal ribosome entry site (internal ribosome entry site, IRES) of encephalitis myocarditis virus and expresses the gene resistant to puromycin, which can make Antisense mRNA of human TGF-β2 is expressed alone in cells, and only cells expressing antisense mRNA of human TGF-β2 can grow stably under the selection of puromycin. The structure of the pIRESpuro3 plasmid is attached figure 1 shown.
[0036] 2. Preparation of stable cell lines
[0037] Collect four non-small cell lung cancer cell lines in logarithmi...
Embodiment 2
[0052] Embodiment 2: Preparation of Ovarian Cancer Tumor Therapeutic Vaccine
[0053] According to the method described in Example 1, the human TGF-β2 antisense plasmid was transfected into: human ovarian cancer cell A2780, human ovarian cancer cell CoC1, human ovarian cancer cell CoC2, human ovarian cancer cell OVCAR-3, human ovarian cancer cell Ovarian cancer tumor therapeutic vaccines were prepared respectively in cell SK-OV-3 and human ovarian cancer cell line SK-OV-3 / DDP tumor cell line.
[0054] In practical application, multiple tumor cell lines after transfection can be selected according to the different tumor antigenicity of patients, so as to adapt to cancer patients with different differentiations.
Embodiment 3
[0055] Embodiment 3: Preparation of breast cancer tumor therapeutic vaccine
[0056] According to the method described in Example 1, human TGF-β2 antisense plasmids were transfected into: human breast medullary carcinoma cell Bcap-37, human breast ductal carcinoma cell BT474, human breast cancer cell MDA-MB-157, human breast carcinoma cell In ductal carcinoma cells MDA-MB-231, human breast cancer cells MDA-MB-453, human breast adenocarcinoma SK-BR-3, human breast adenocarcinoma MCF-7 tumor cell lines, breast cancer tumor therapeutic vaccine.
[0057] In practical application, multiple tumor cell lines after transfection can be selected according to the different tumor antigenicity of patients, so as to adapt to cancer patients with different differentiations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com