AIDS vaccine and preparation method thereof
A technology of AIDS and vaccines, applied in the field of genetic engineering, can solve the problems of high cost, high price, and limited effect, and achieve the effects of saving time, easy cultivation, and rapid growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The construction of embodiment 1 recombinant plasmid
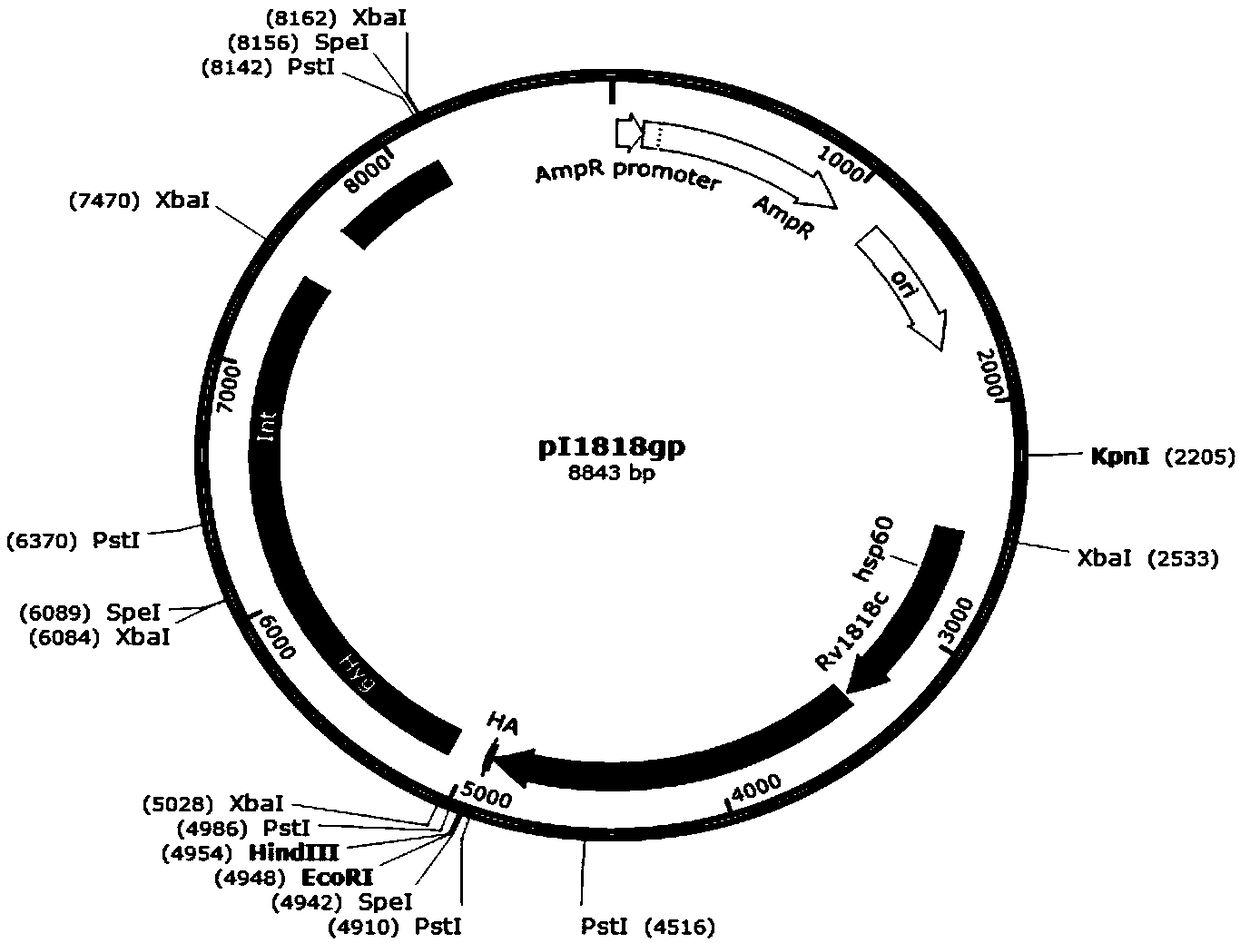
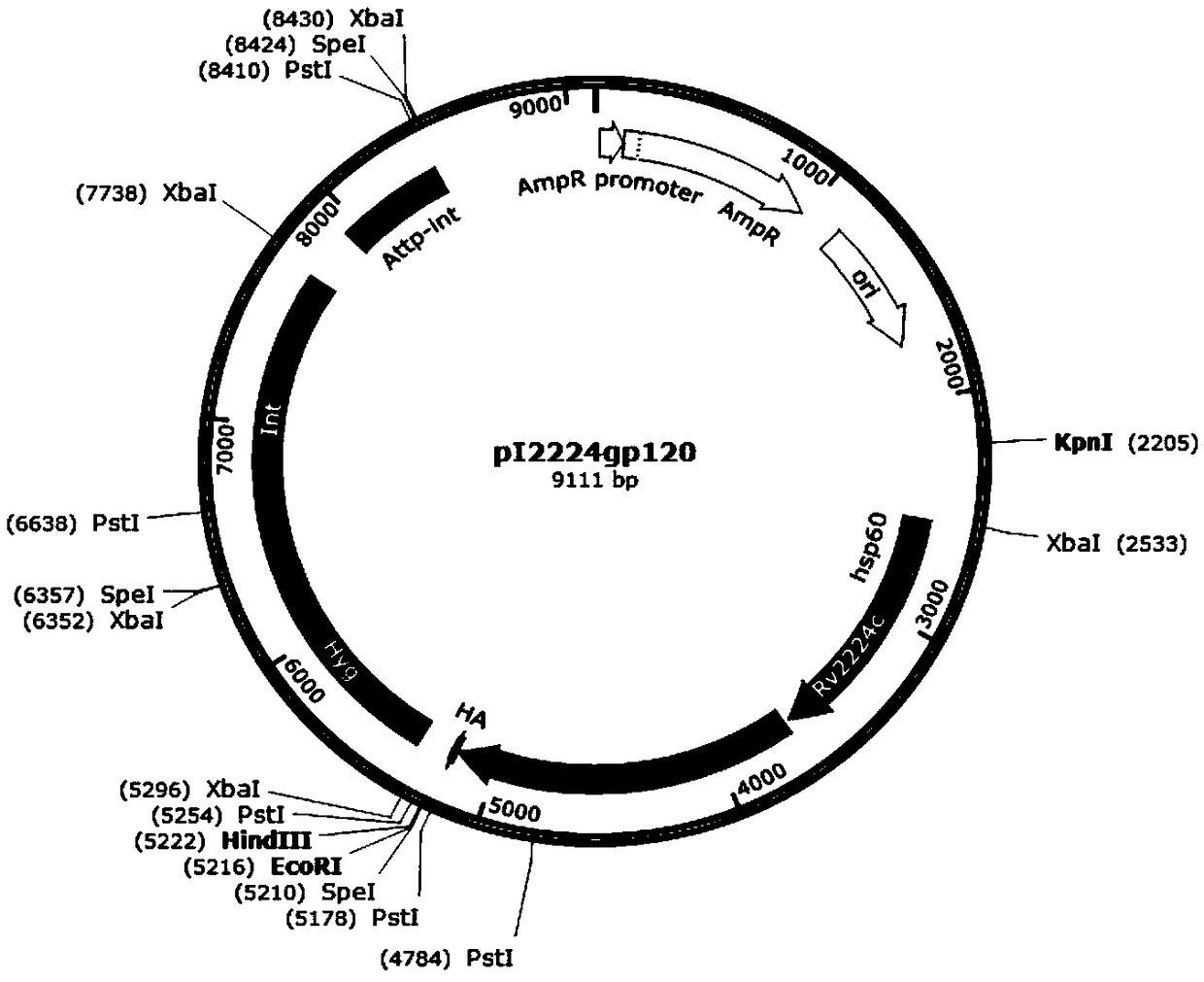
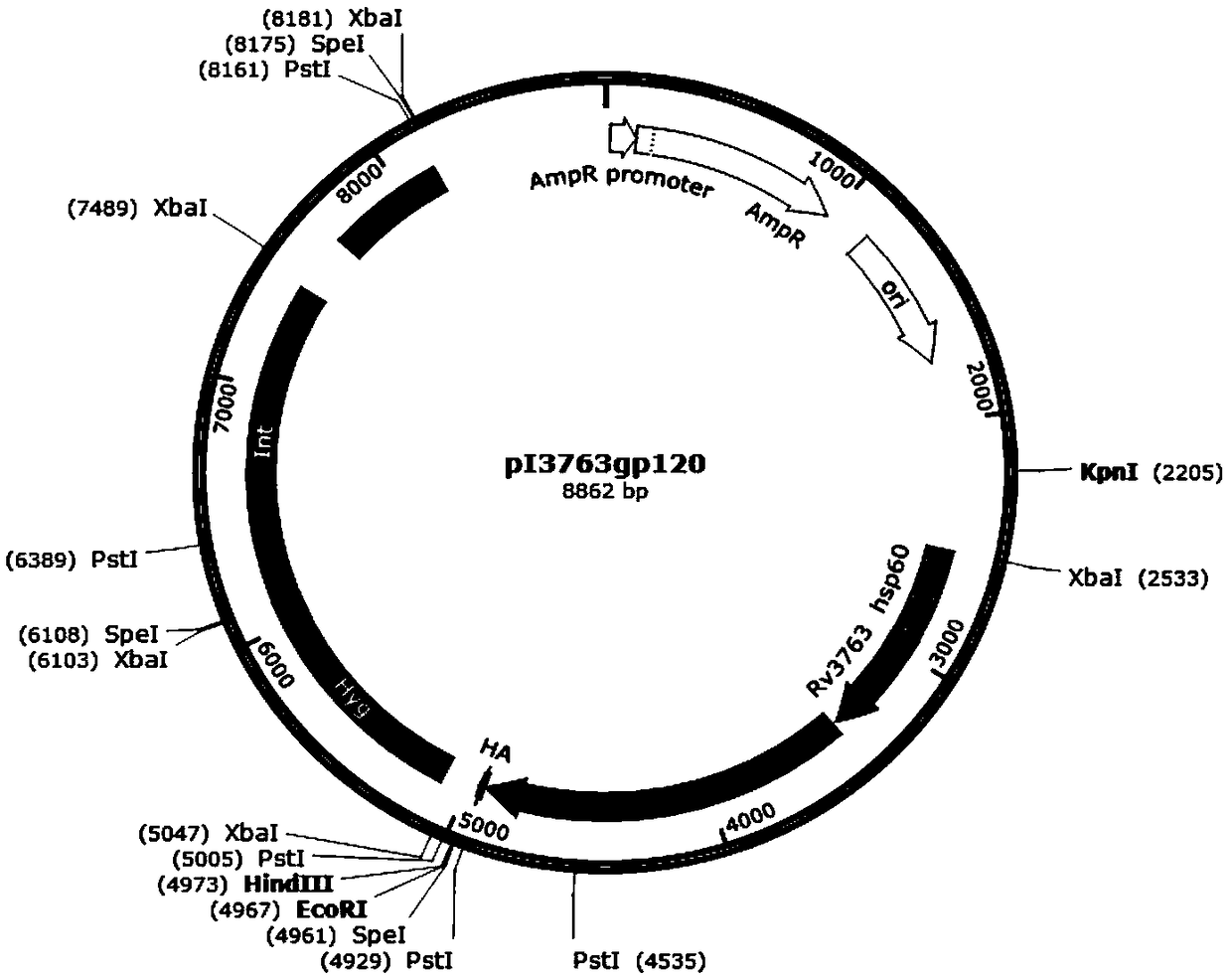
[0041] (1) Construction of integrated plasmid pI1818gp, plasmid pI2224gp, plasmid pI3763gp and intracellular control plasmid pIgp, the structure diagrams are shown in figure 1 , figure 2 , image 3 and Figure 4 , see the construction process respectively Figure 4 and Figure 5 (The construction flowchart of plasmid pI2224gp and plasmid pI3763gp can be deduced by analogy). Depend on figure 1 As shown, plasmid pI1818gp contains resistance selection gene AmpR, Escherichia coli replicon oriE, promoter hsp60, carrier protein gene Rv1818c, Linker sequence, antigen gene gp120 derived from simian immunodeficiency virus SIV, and human influenza blood in a clockwise direction. The lectin virus gene tag HA-tag, the fusion gene of the resistance screening gene Hyg and the integrase gene Int, and the fusion gene of the phage integration site Attp and the integrase gene Int; figure 2 As shown, the plasmid pI2224gp has ...
Embodiment 2
[0071] The construction of embodiment 2 recombinant Mycobacterium smegmatis
[0072] The relevant plasmid constructed in Example 1 was transformed into Mycobacterium smegmatis ZWY2, and the expression of the antigenic protein gene gp120 in each strain was verified by western-blot, and it was expressed on the surface of Mycobacterium smegmatis ZWY2 strain. The specific operation is as follows:
[0073] 1. Conversion:
[0074] The plasmids pI1818gp, pI2224gp, pI3763gp and pIgp were transformed into Mycobacterium smegmatis ZWY2 by electroporation (see reference 18 for the transformation method), and corresponding transformants were obtained, which were respectively named as pI1818gp strain, pI2224gp strain, The pI3763gp strain and the pIgp strain, wherein, the pI1818gp strain, the pI2224gp strain, and the pI3763gp strain are AIDS vaccines.
[0075] 2. Western-blot verification of the surface display of the antigenic protein gene gp120:
[0076] Firstly, the subcellular compone...
Embodiment 3
[0081] Embodiment 3 AIDS vaccine immunogenicity test
[0082] Evaluate the immunogenicity of embodiment 2 AIDS vaccine by animal experiments, 84 6-week-old C57BL / 6 female mice purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. were randomly divided into 7 groups, respectively pI1818gp strain group, pI2224gp strain group, pI3763gp strain group, pIgp strain group, ZWY2 group, PBS group (negative control) and adenovirus vector Ad5SIV-env group (positive control, gifted by researcher Chen Ling, Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences), each The number of animals in the group is shown in Table 1. The IACUC number of this animal experiment is 2017024.
[0083] Table 1 Mouse grouping and immunization rules
[0084]
[0085] The specific operation is as follows:
[0086] 1) Cell preparation and immunization, the specific operation is as follows:
[0087] a. Inoculate the bacterial vaccines pI1818gp strain, pI2224gp st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com