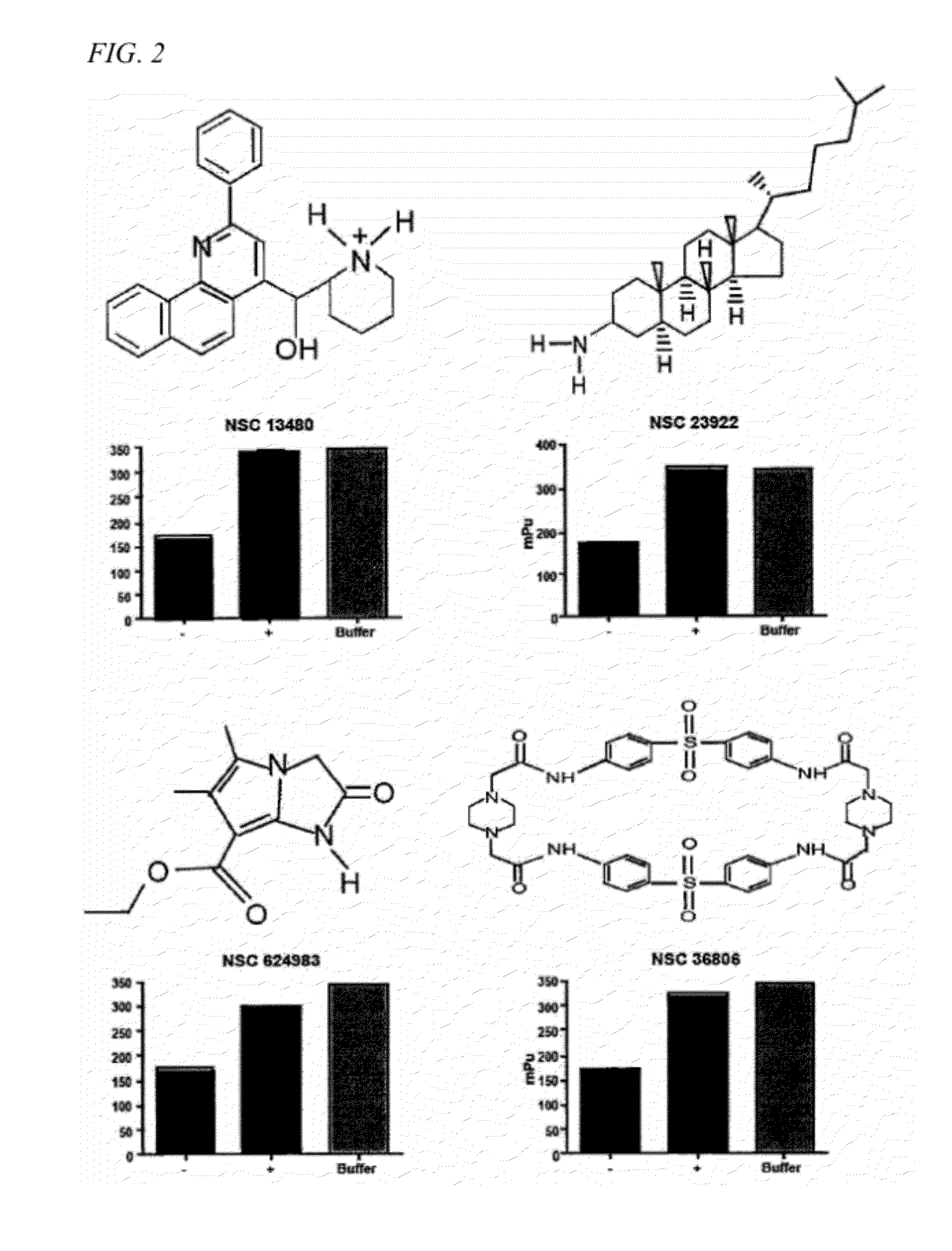
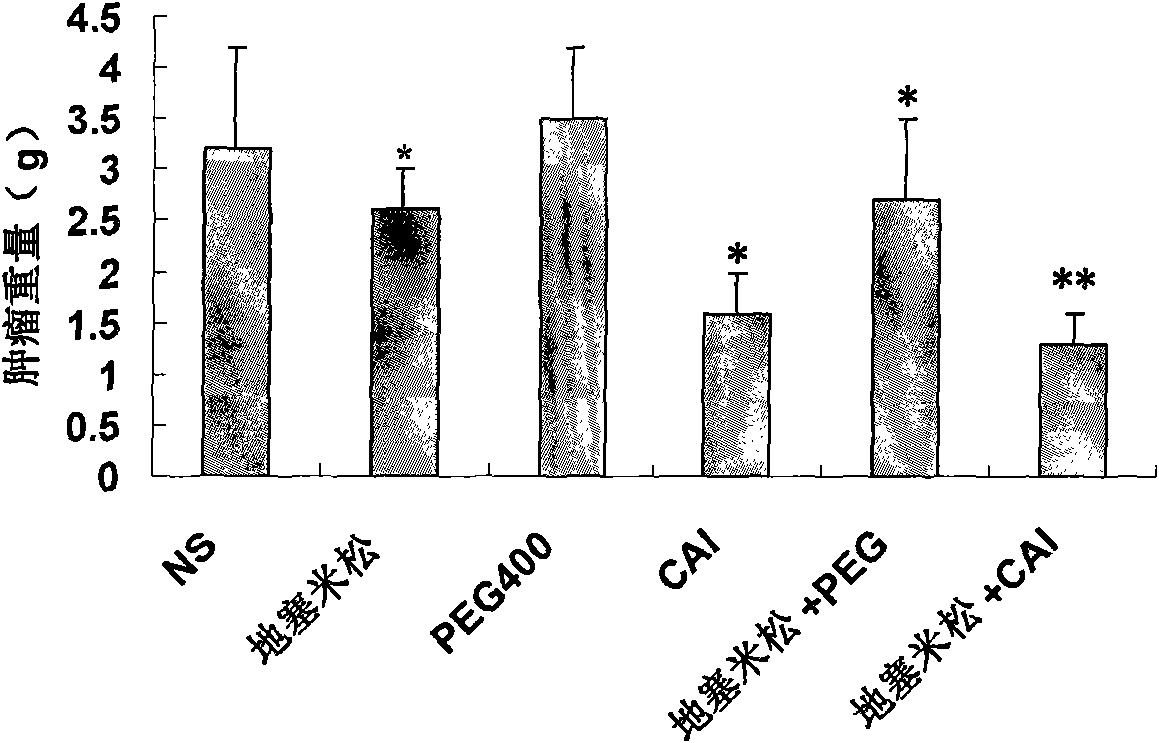
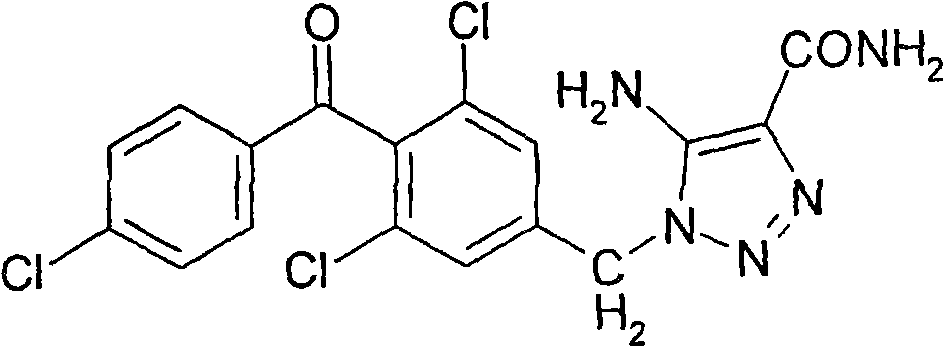
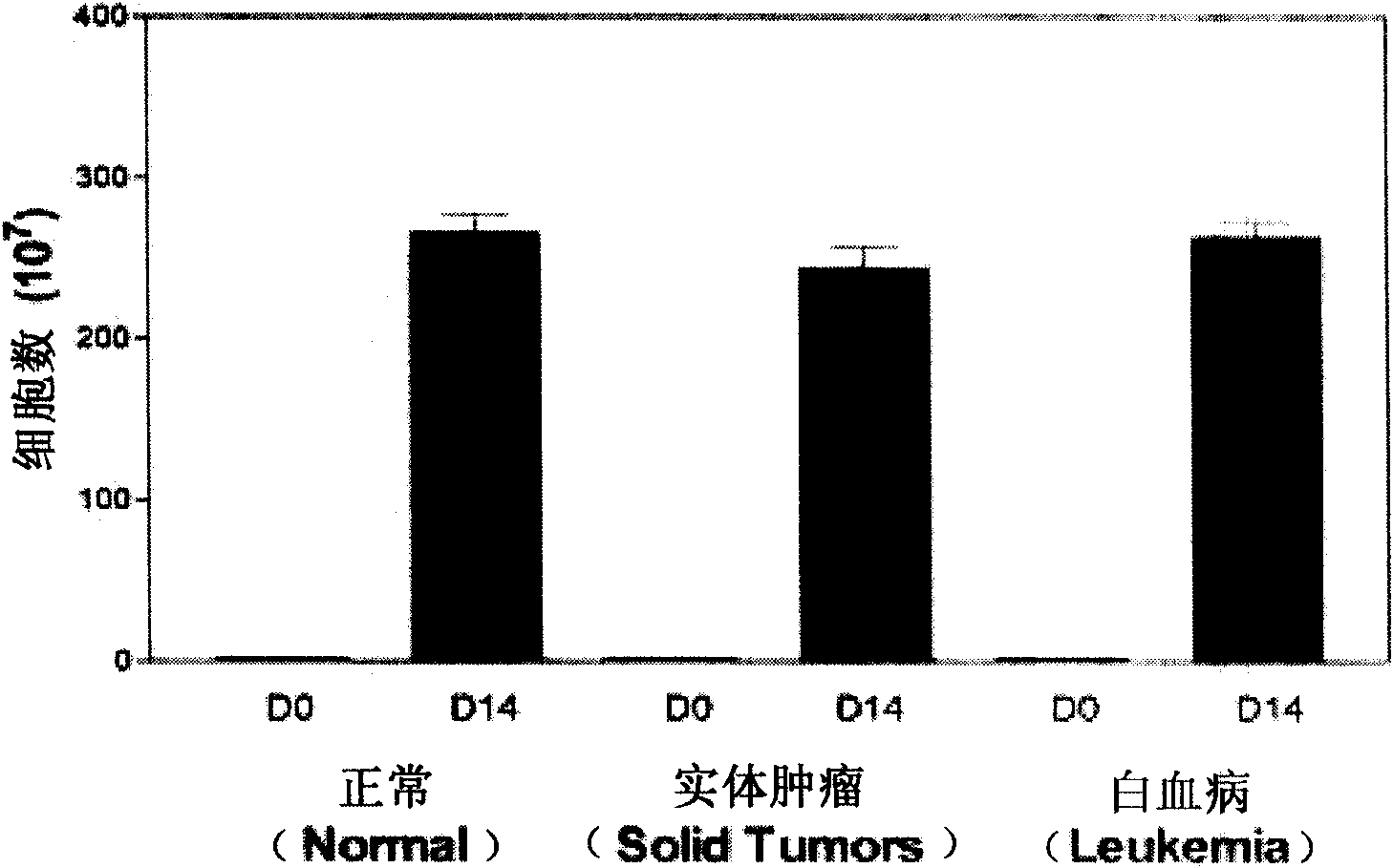
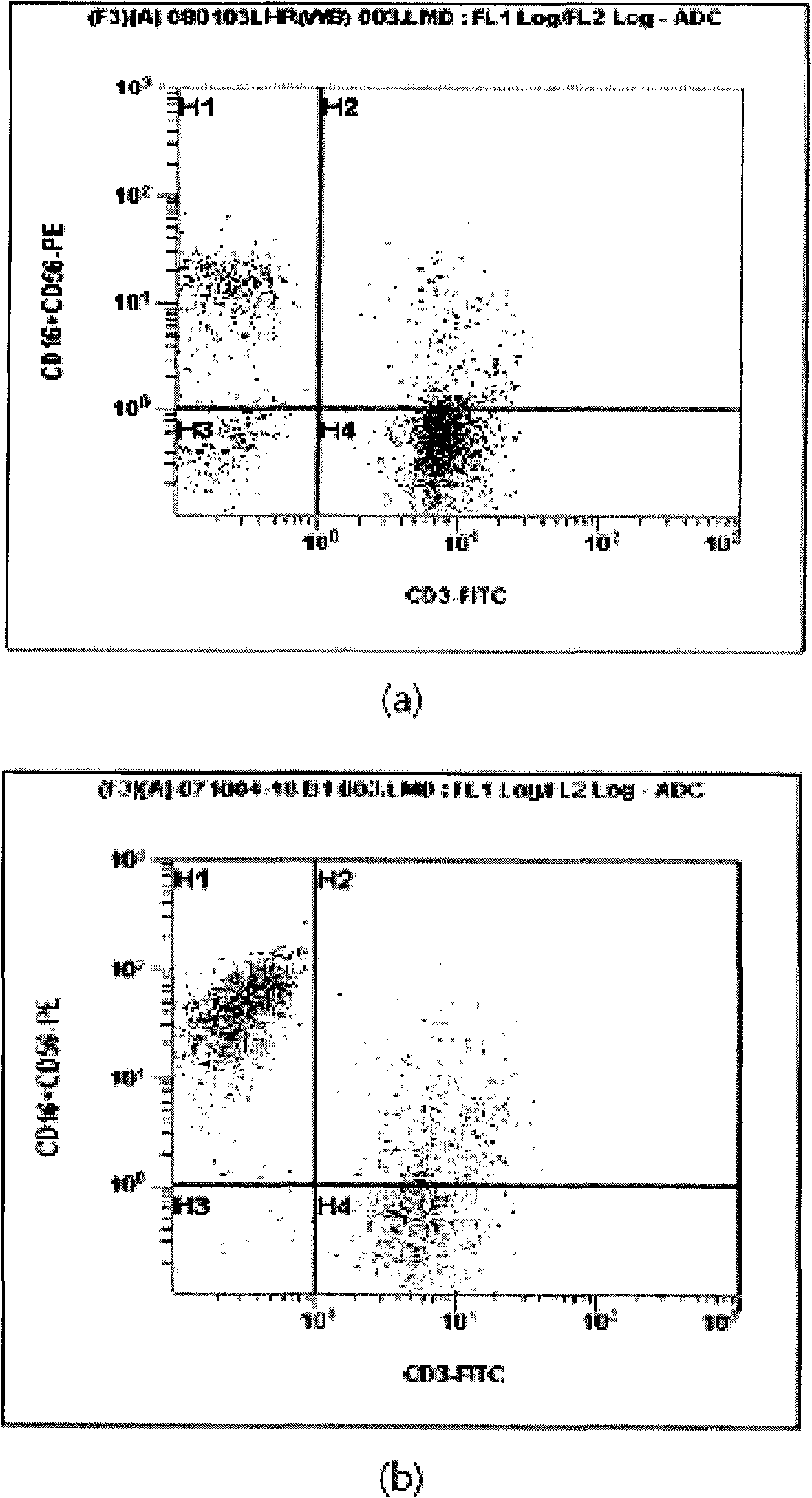
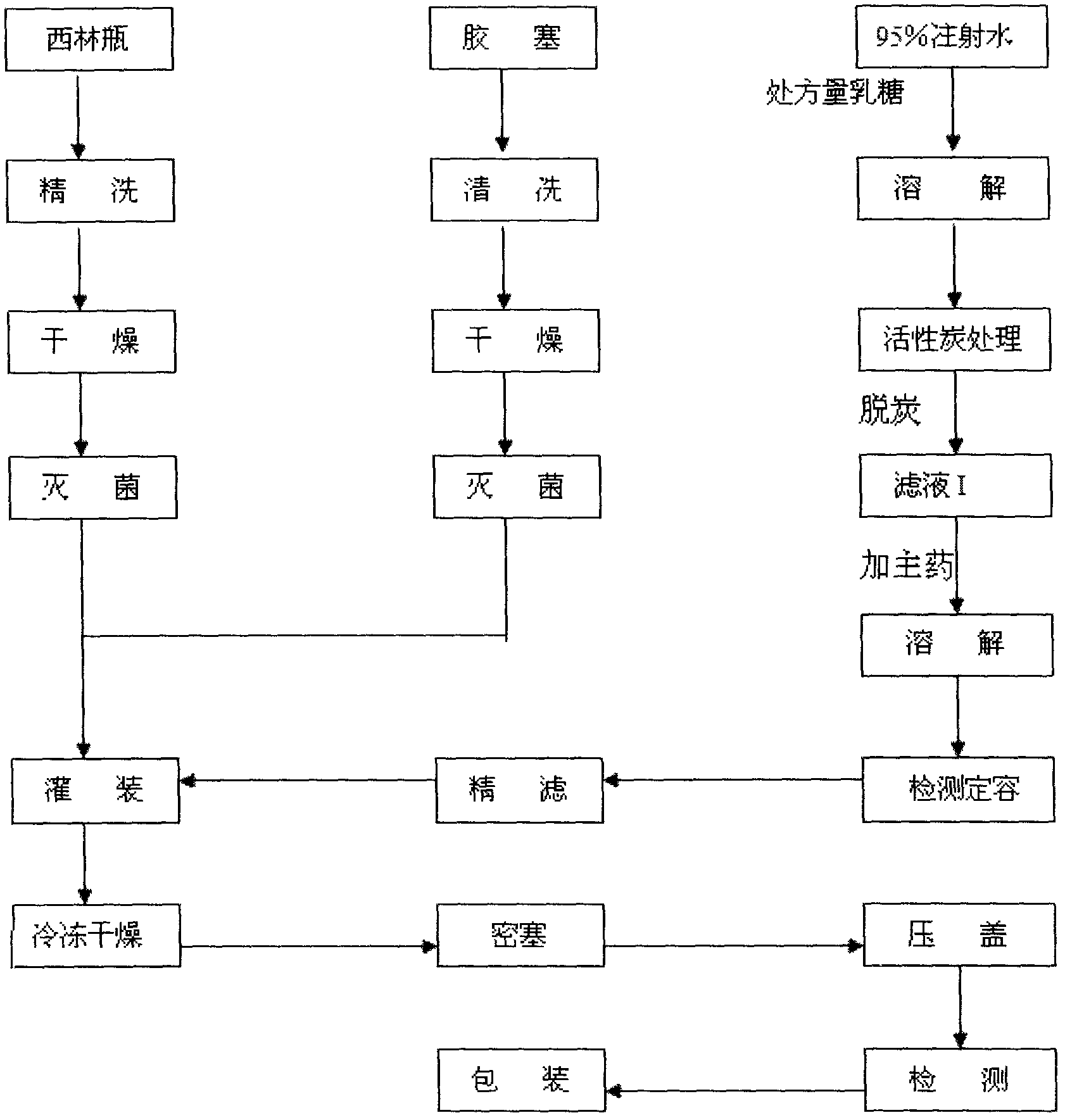
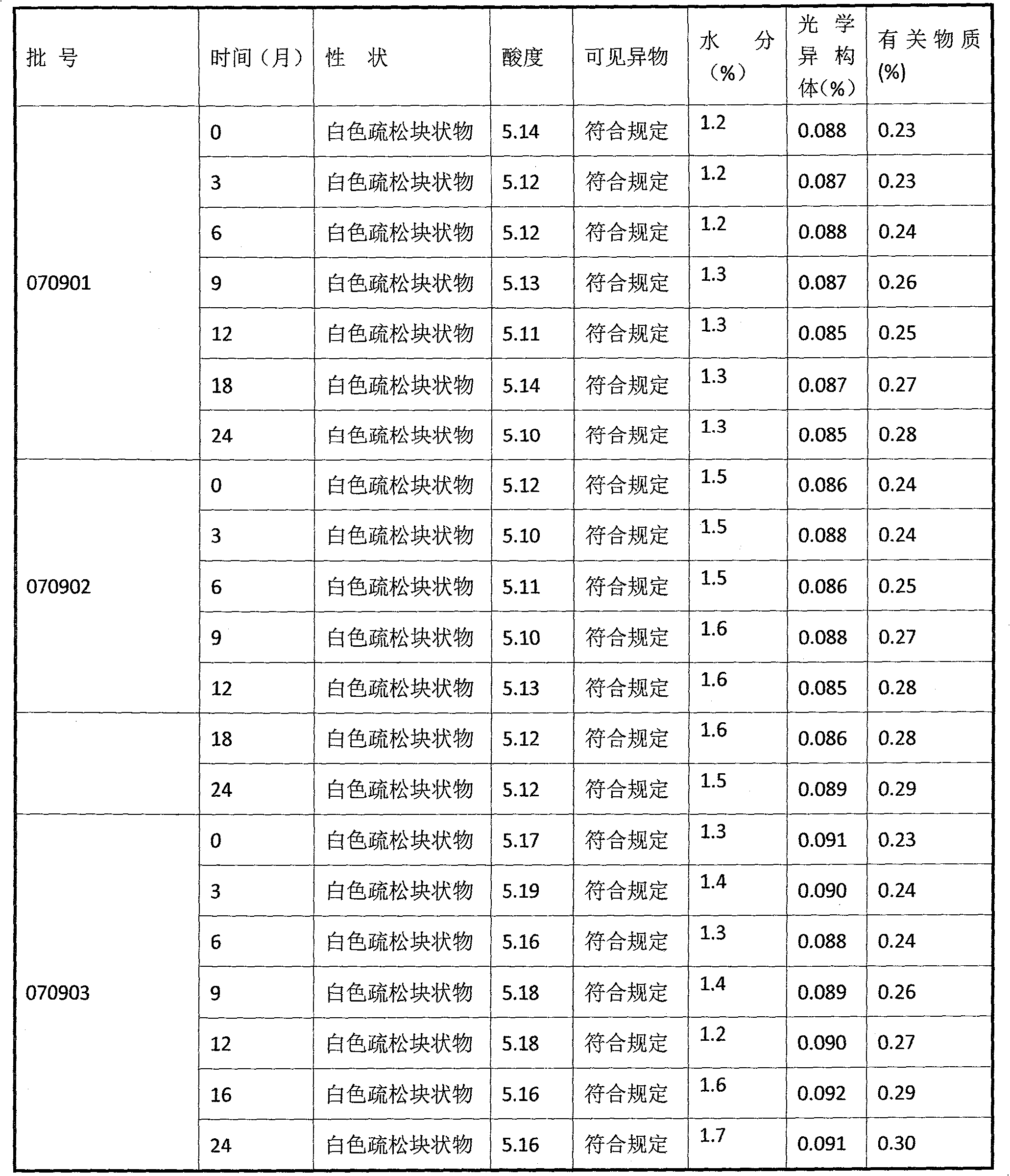
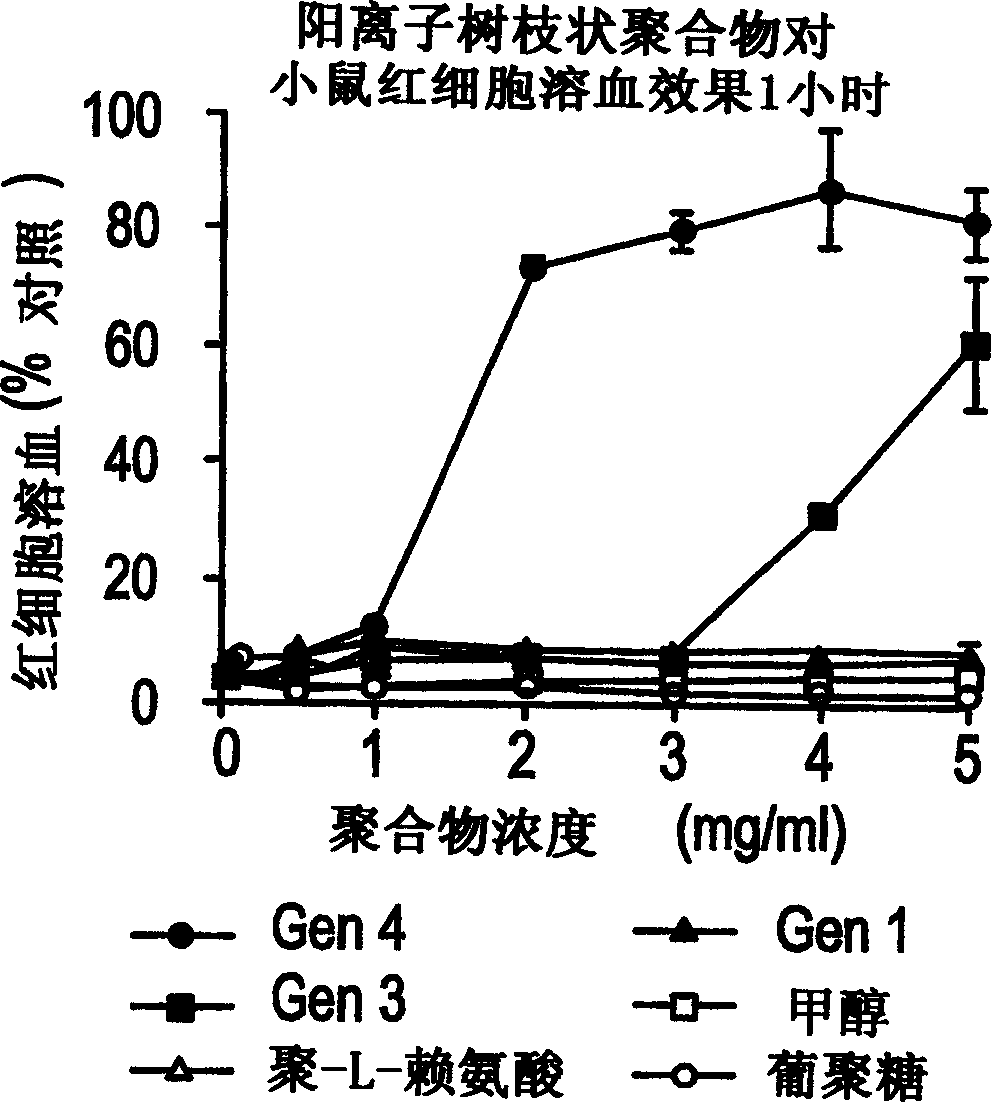
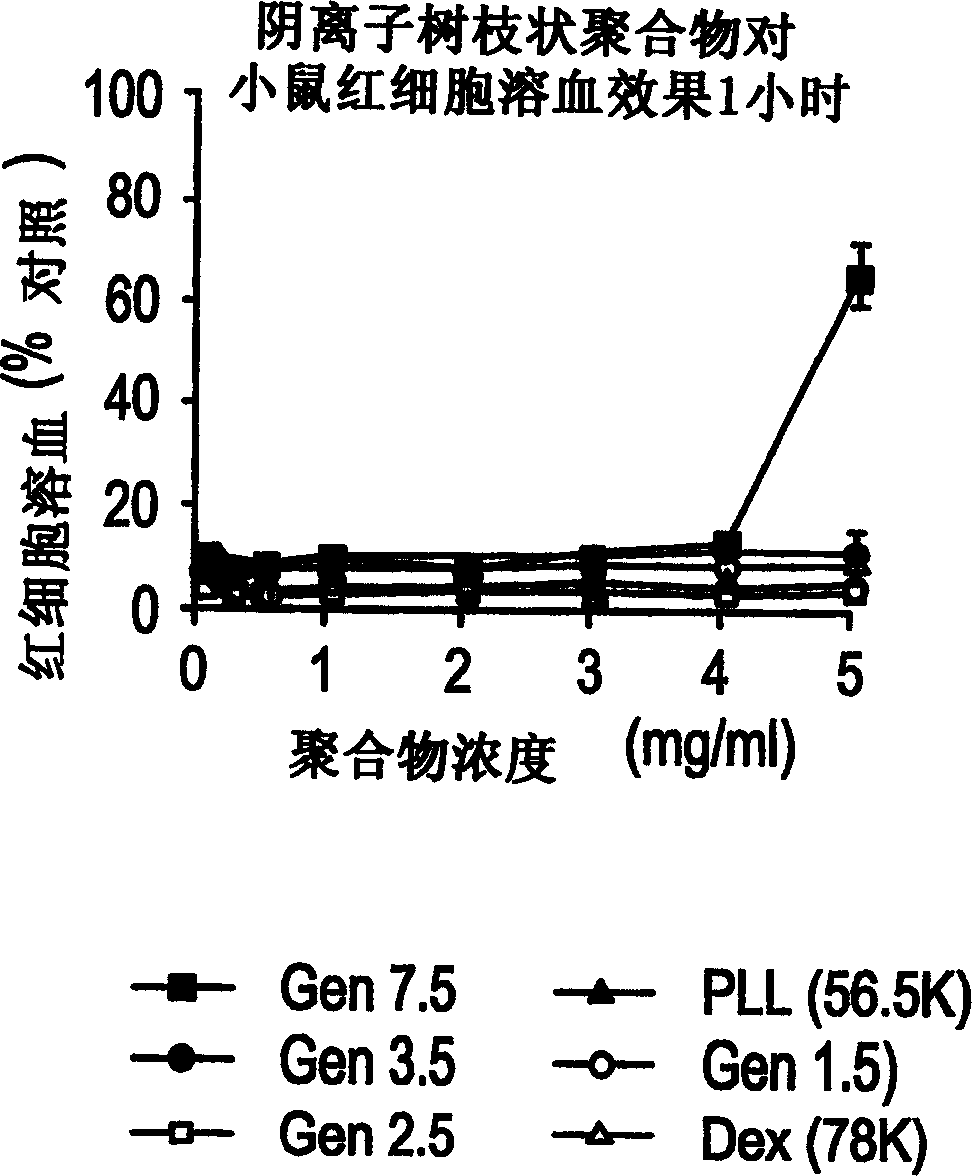
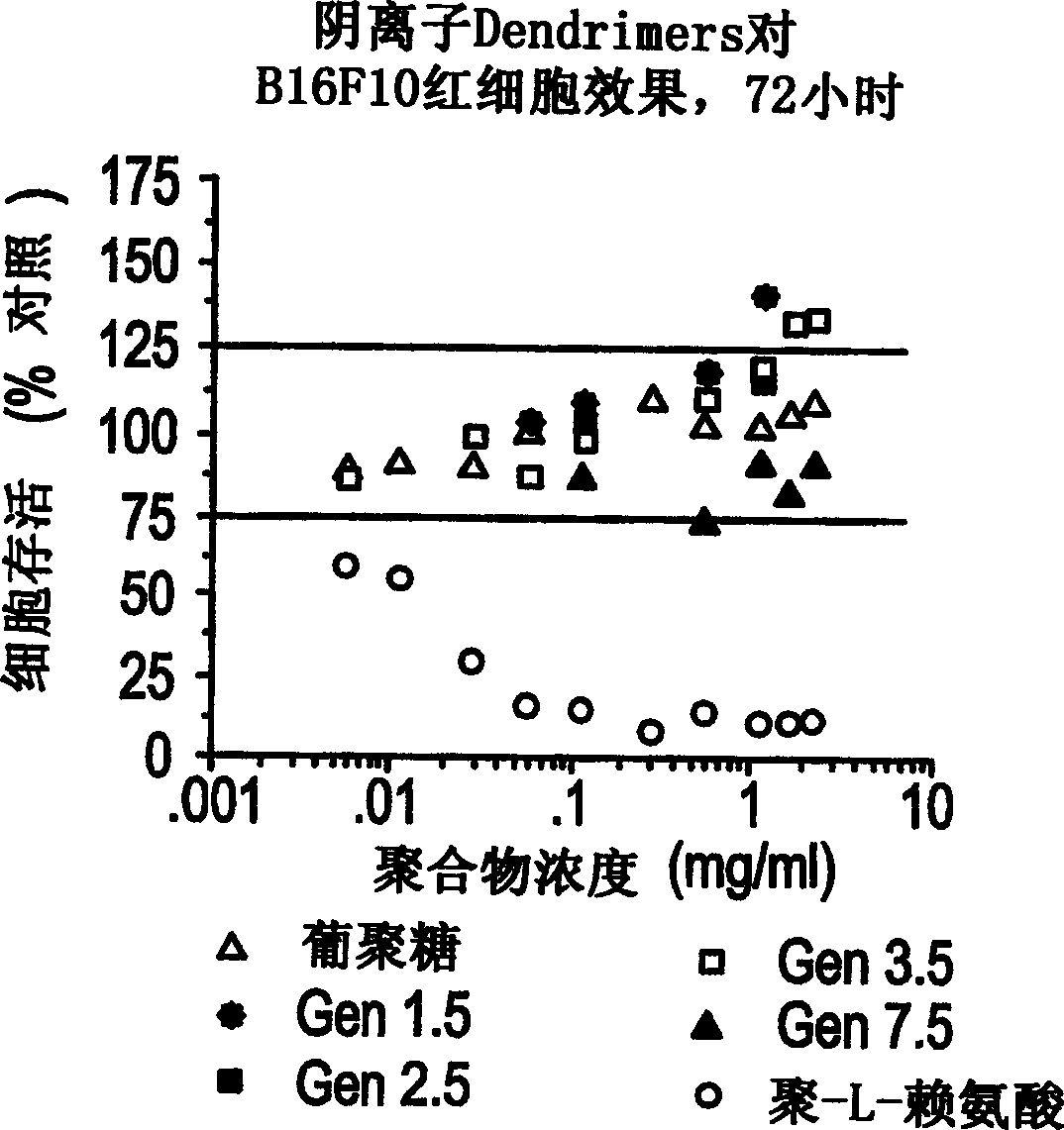
Patents
Literature
113 results about "Malignant Neoplasm Treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treatments for Malignant Neoplasm (Cancer) include: Treatments depend on the specific type of cancer but some types of treatments are common. Chemotherapy. Radiation therapy. Surgery. Biological therapy (immunotherapy)
Antigenic polypeptide usable as therapeutic agent for malignant neoplasm
ActiveUS8323657B2Promote productionTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeNeoplasm
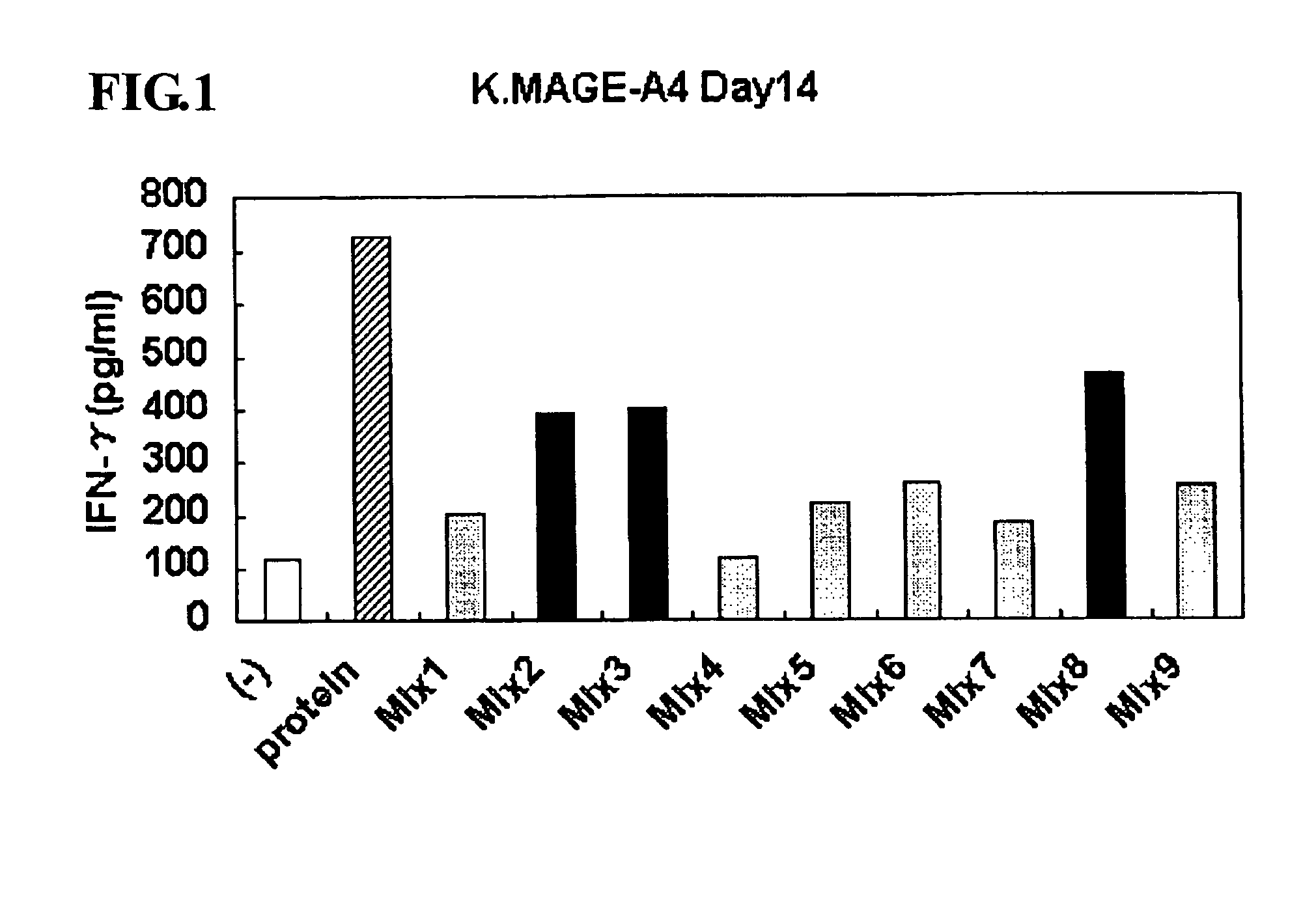
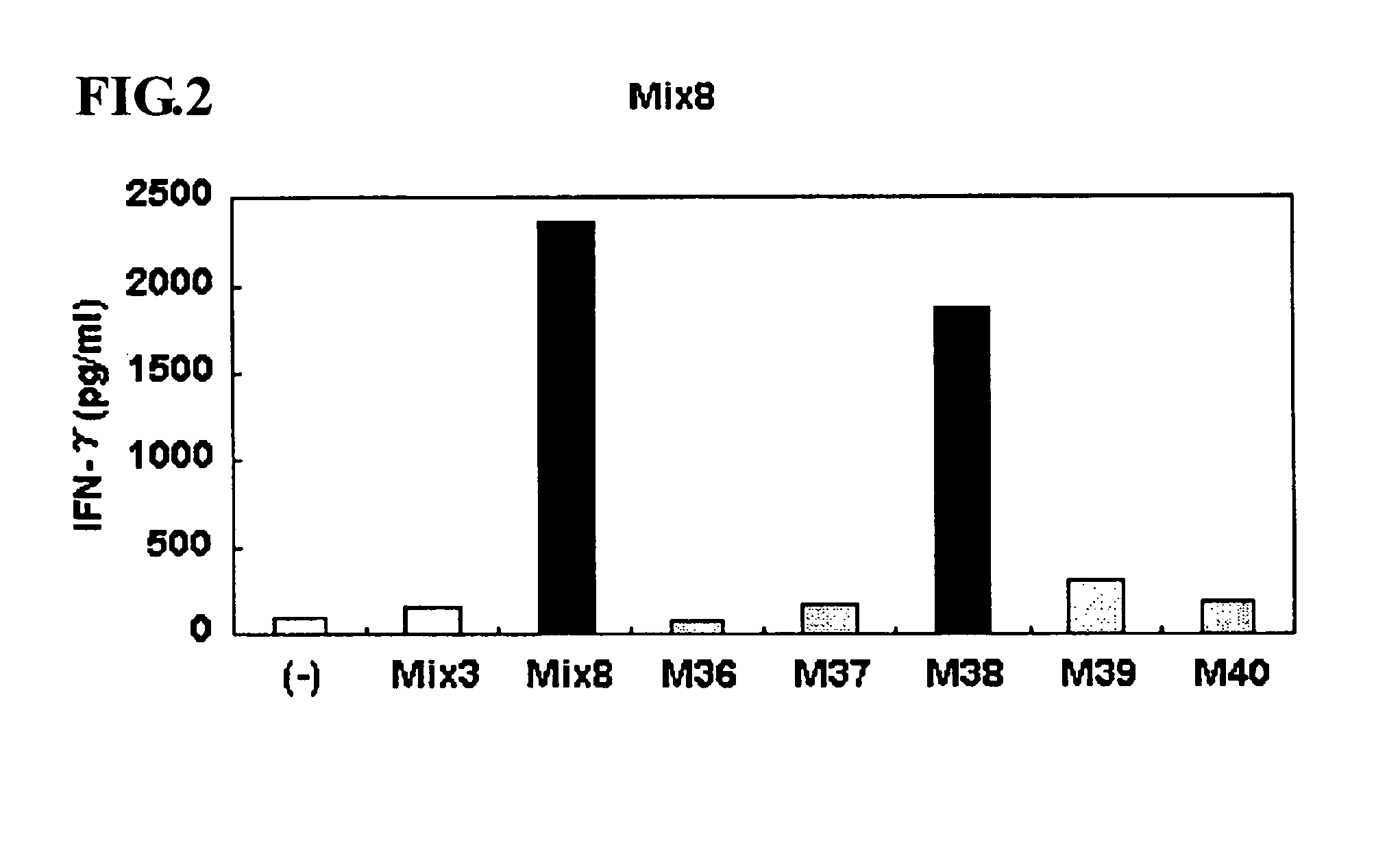
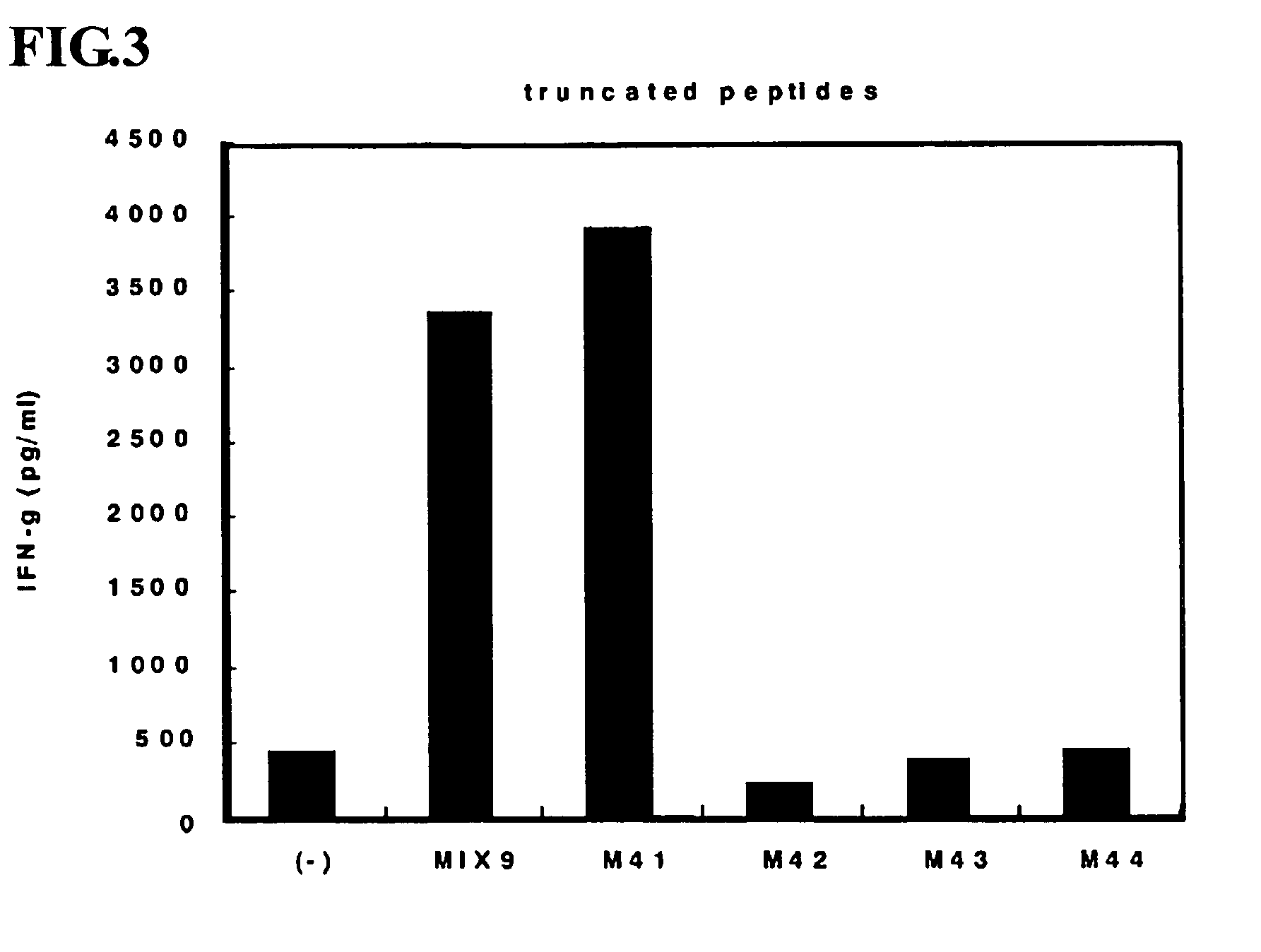
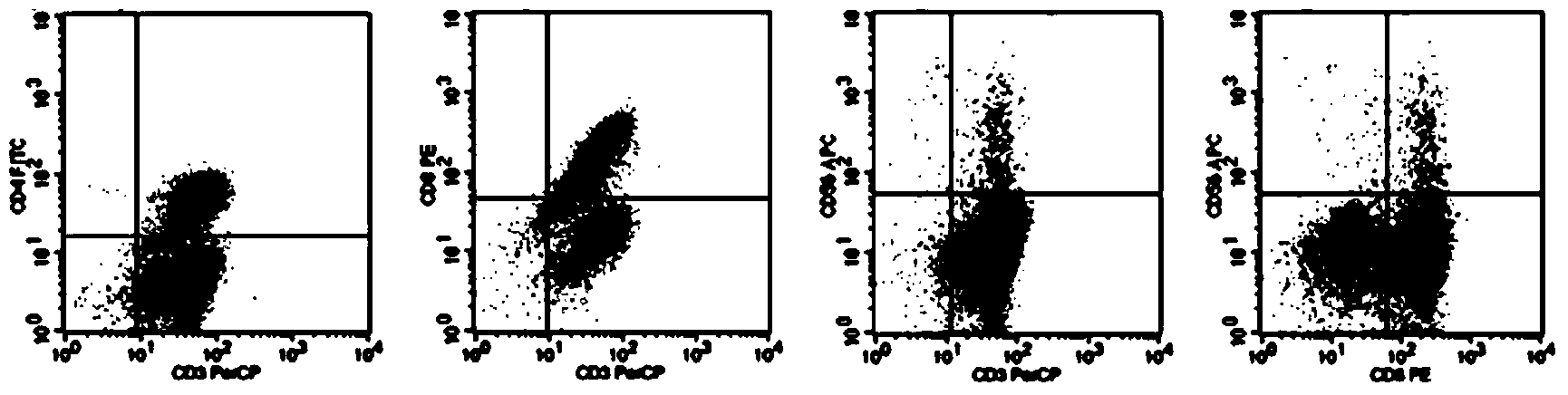
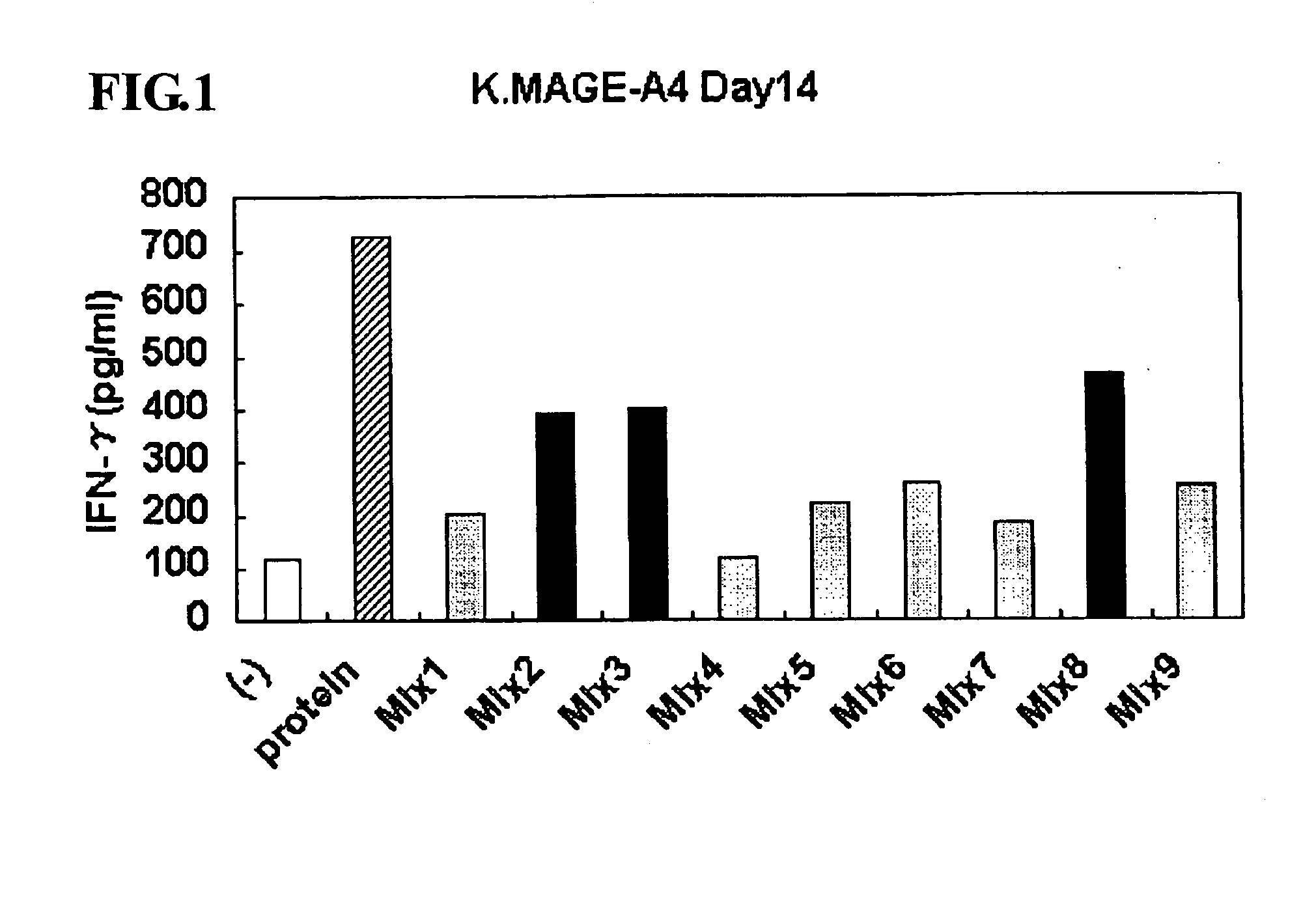
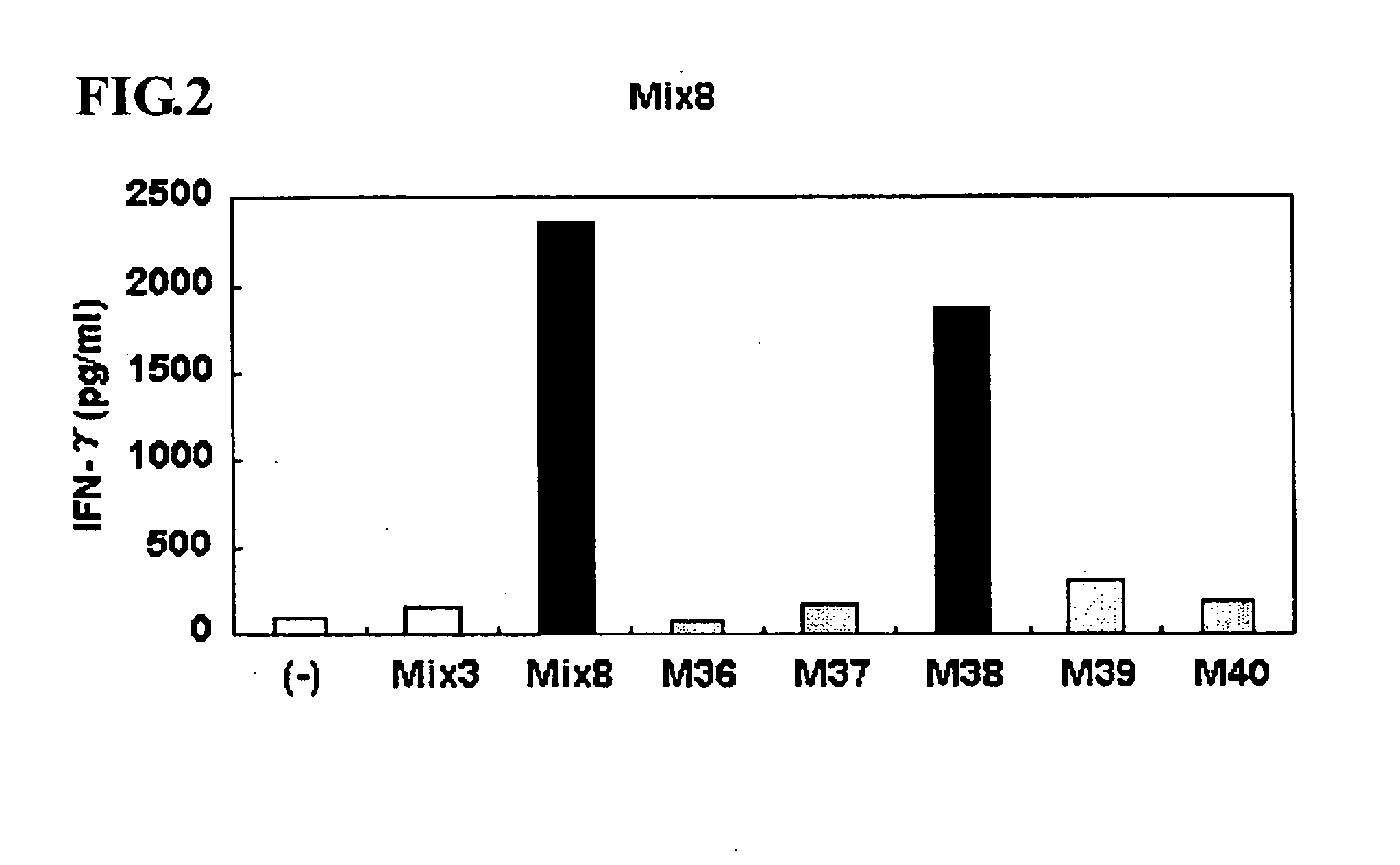
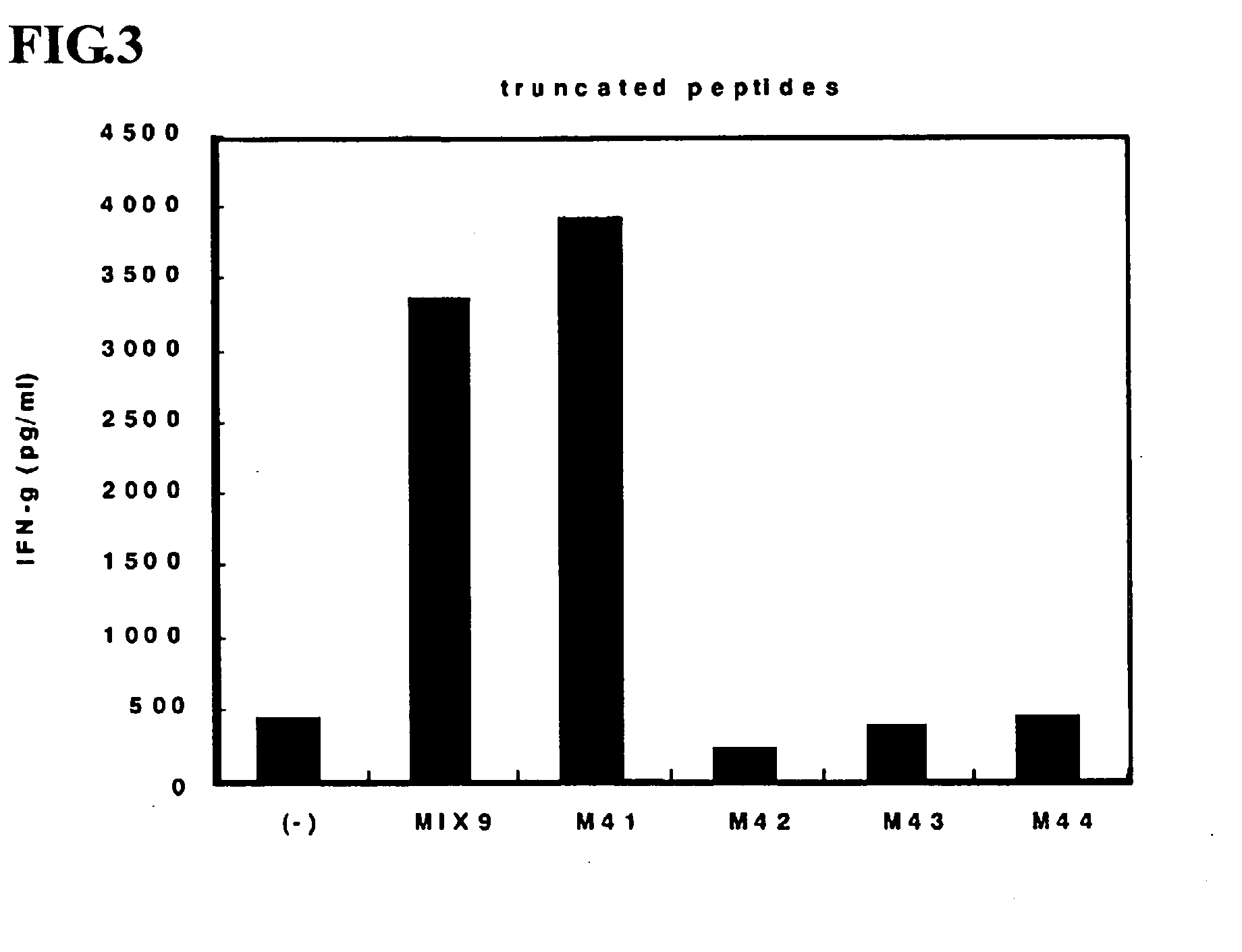
This invention provides a new tumor antigen having an epitope that induces a Th1 cell (a CD4-positive T cell specific to MAGE-A4), and a method ot application thereof.
Owner:HOKKAIDO UNIVERSITY
Engineered CD20 targeting NKT cell and its preparation method and application
ActiveCN103820393AHigh transfection rateProlong survival timePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD20Tumor antigen
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Method of modulating ship activity
InactiveUS20120178725A1Easy to optimizeMobilizeBiocideMetabolism disorderGraft versus host disease inductionAllogeneic transplantation
A method of treating or preventing an immune disorder, such as graft versus host disease, in a subject. The method includes the administering a SHIP1 inhibitor, such as 3α-aminocholestane, to a subject in need of treatment. Thus, SHIP1 inhibitors taught herein represent a novel class of small molecules that have the potential to enhance allogeneic transplantation, boost innate immunity and improve the treatment of hematologic malignancies.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
Graphene oxide drug carrier as well as preparation method and application thereof
InactiveCN103110957AEasy to makeNo chemical reactions involvedMacromolecular non-active ingredientsAntineoplastic agentsCytotoxicityBiocompatibility Testing
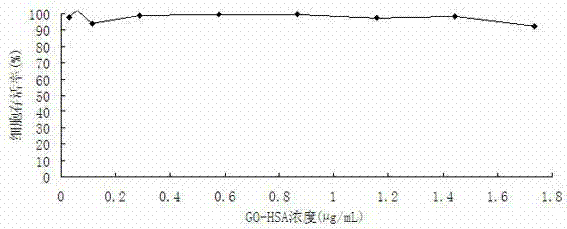
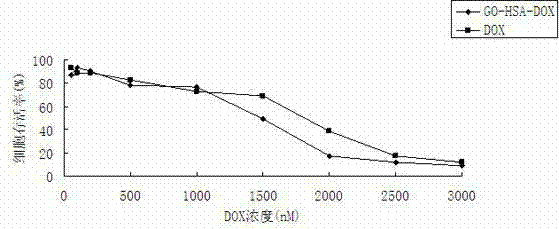
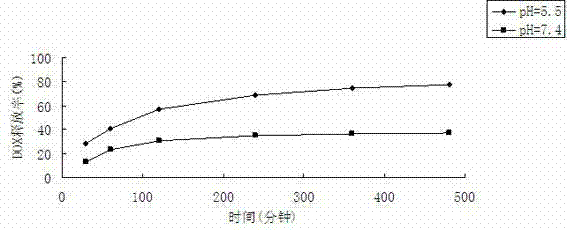
The invention discloses a graphene oxide drug carrier as well as a preparation method and an application thereof. A monolayer or multilayer graphene oxide of human serum albumin is combined to serve as a transport carrier of the drug, and active ingredients of the drug are loaded by absorption. The drug carrier is simple in preparation, good in biocompatibility and very low in cytotoxicity, so that the drug carrier has a wide application prospect in a clinical application, and particularly in malignant tumor treatment aspect.
Owner:FUZHOU UNIV
Gene chip used for detecting related gene mutations of malignancy individual medications and application thereof
InactiveCN101619350AAccurate detectionSensitive detectionMicrobiological testing/measurementMalignancyWilms' tumor
The invention relates to a gene analysis detecting product, in particular to a gene detecting chip and a reagent matched with the same, more particular to a gene detecting chip and a matched reagent used for detecting a series of gene mutations that are closely related to the reactiveness of medicines for treating malignancy as well as the application thereof. The gene chip can detect known gene mutations closely related to individual difference of anti-tumor medicine reaction in comprehensive, systemic and high-flux ways by selecting appropriate mutant sites and designing appropriate primers and probes and can work out corresponding adjusting schemes aiming at different medicines.
Owner:湖南宏灏基因生物科技有限公司
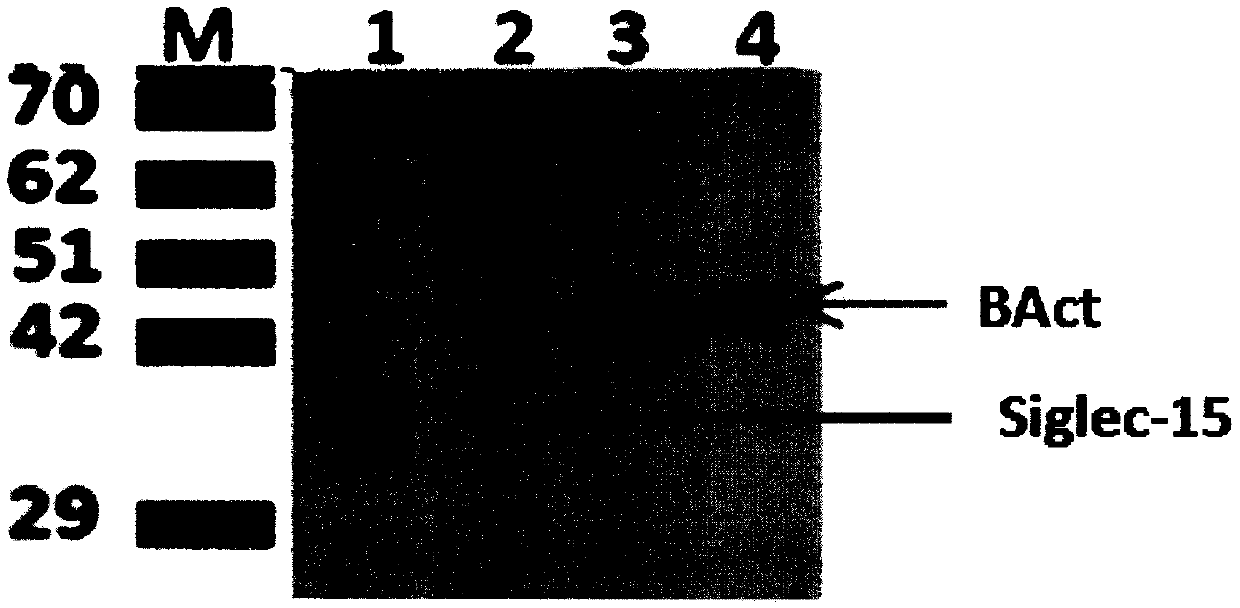
Preparation and application of mouse monoclonal antibody against human Siglec-15 protein
InactiveCN110386982AImprove featuresImprove reliabilityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPD-L1Wilms' tumor
The invention discloses a mouse monoclonal antibody against a human Siglec-15 protein. The specificity is high, the performance is stable and the titer is high. The invention further relates to application of the monoclonal antibody to preparation of immunohistochemical detection tools for the Siglec-15 protein. According to the mouse monoclonal antibody against the human Siglec-15 protein, an immunohistochemistry method is used for detecting expression of Siglec-15 on the tumor cell surface for selection, the curative effect and the prognosis of therapeutic schedules of malignant tumors. Theinvention further relates to the application of the monoclonal antibody to preparation of antibody drugs for immunotherapy of the malignant tumors. The important application value is achieved. The adopted antibody drugs can be full-length or partial sections (Fab, F(ab)'2 or ScFv) of an Siglec-15 monoclonal antibody or combined use of the Siglec-15 monoclonal antibody and a PD-L1 monoclonal antibody. The malignant tumors mainly include colorectal cancer, endometrial cancer, thyroid cancer, bladder cancer, kidney cancer, lung cancer, liver cancer and the like.
Owner:广东三九脑科医院 +1
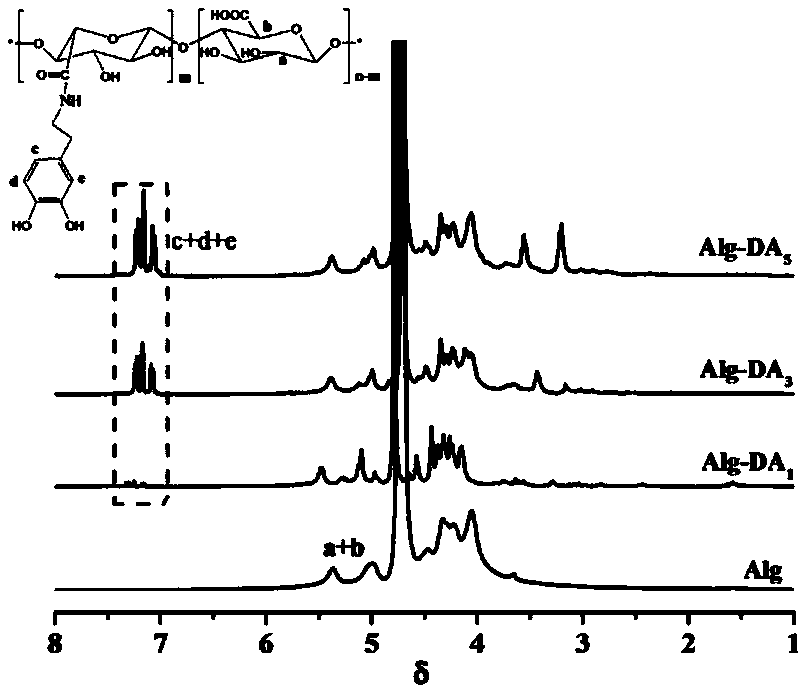
Preparation method of injectable hydrogel with photo-thermal property
ActiveCN107583049AInjectableWith photothermal propertiesEnergy modified materialsAerosol deliveryMedicineSodium glycerophosphate
The invention discloses a preparation method of an injectable hydrogel with a photo-thermal property, and belongs to the field of functional materials. In a malignant tumor treatment, a patient shouldtake medicines for many times, while provided hydrogel only needs one injection. The preparation method comprises the following steps: at first, preparing a dopamine modified sodium alginate bio-macromolecule (Alg-DA); adjusting the gelation temperature of a temperature sensitive hydrogel (CGP) of chitosan (CS) and sodium beta-glycerophosphate (beta-GP) to the range of human physiological temperature so that the hydrogel is in a sol state at a room temperature and will become gel after arriving at a human body; then mixing Alg-DA and CGP to obtain an injectable temperature sensitive hydrogel,and doping a polydopamine wrapped gold nano rod (Au-PDA NRs), which is prepared in advance, into the hydrogel to obtain the injectable hydrogel with a photo-thermal property (CGP / Alg-DA@Au-PDA). Thehydrogel can fix Au NRs in tumors and carries out multiple photo-thermal conversions under laser radiation so as to treat tumors by utilizing high temperature. The injectable hydrogel has a potentialapplication value in the biomedicine field.
Owner:JIANGNAN UNIV
Engineering CD20 targeting NKT cell and preparation method and application thereof
ActiveCN106279434AHigh transfection rateProlong survival timePolypeptide with localisation/targeting motifImmunoglobulin superfamilyCD20Antigen receptors
The invention relates to an engineering CD20 targeting NKT cell and a preparation method and application thereof. The cell is an NKT cell modified by chimeric antigen receptor CD20ScFv-CD8-CD137-CD3 sigma, an amino acid sequence of the chimeric antigen receptor is as shown by SEQID NO. 1 in a sequence list. The preparation method comprises the following steps: firstly establishing a chimeric antigen receptor pWPT-CD20ScFv-CD8-CD137-CD3 sigma, infecting the NKT cell, performing in-vitro induction and multiplication culture, and obtaining the engineering CD20 targeting NKT cell. When the engineering CD20 targeting NKT cell is used for treating a CD20 positive malignant tumor at a progressive stage, the survival time of the immune cell in the body of a patient can be obviously prolonged, the capacity of the immune cell for target identifying a tumor antigen is improved, and the killing activity for the tumor cell is improved.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
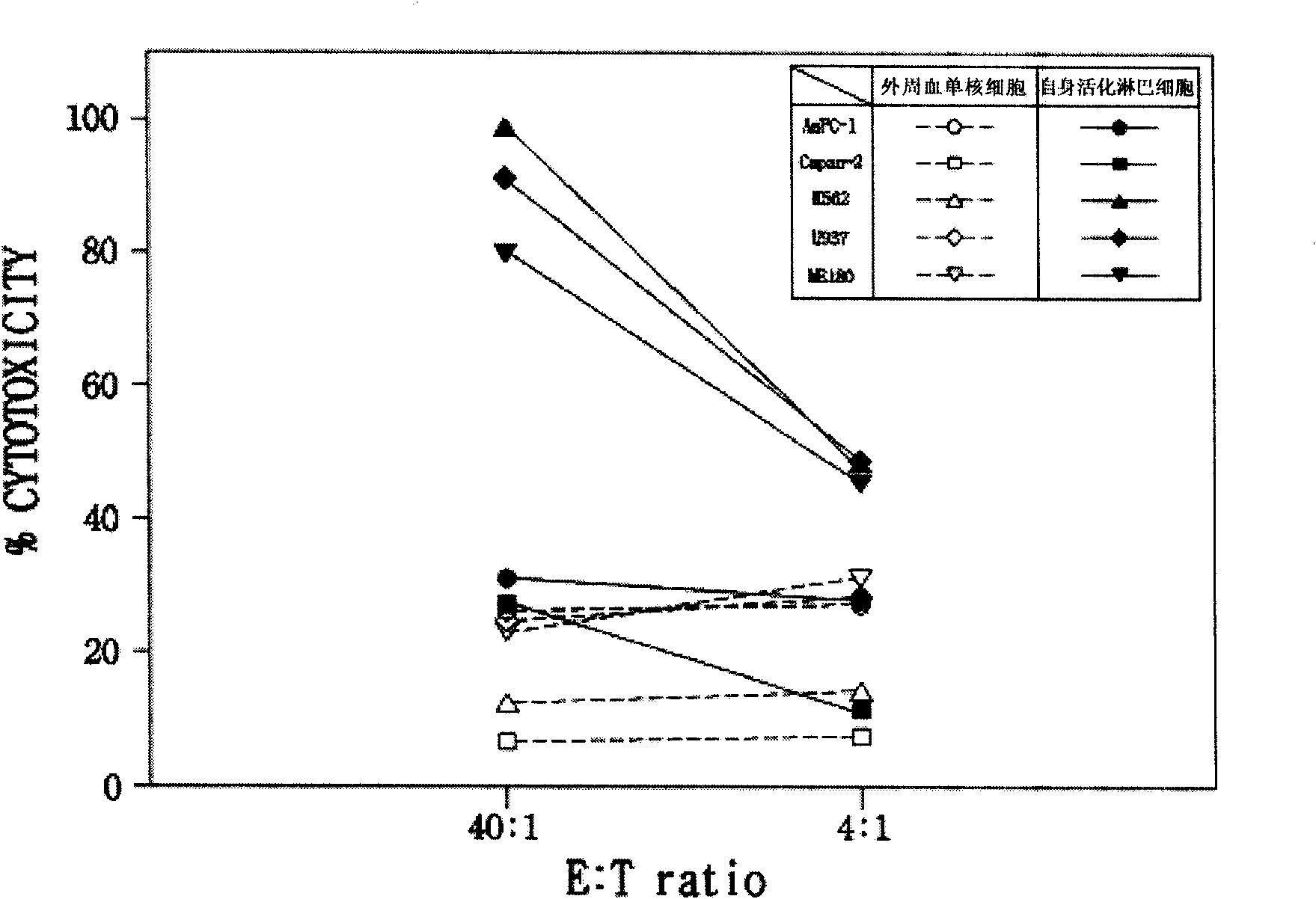
A method for cultivating self activated lymphocyte
InactiveCN101603029ALittle side effectsImproved prognosisCancer antigen ingredientsBlood/immune system cellsCancer cellCD16
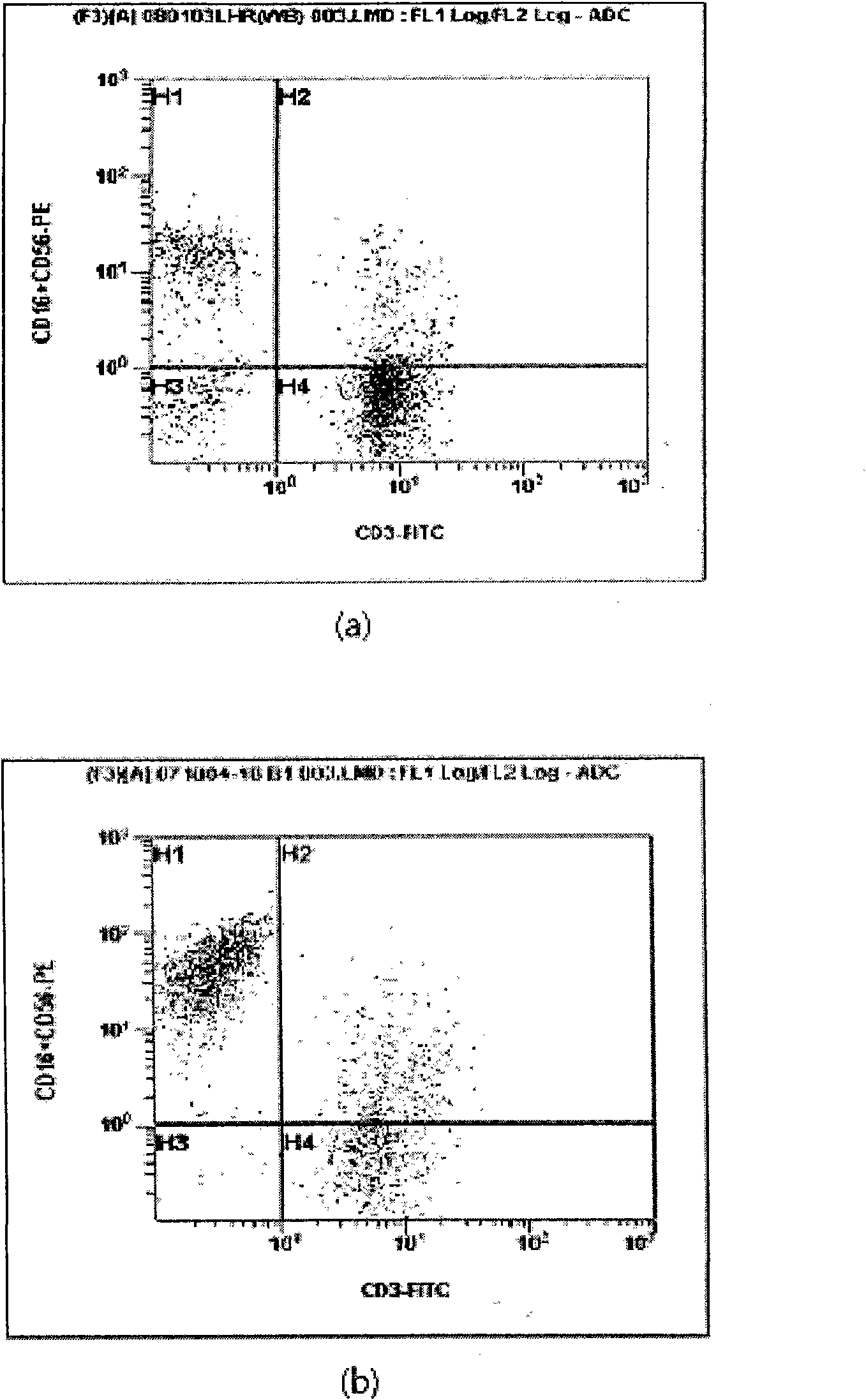
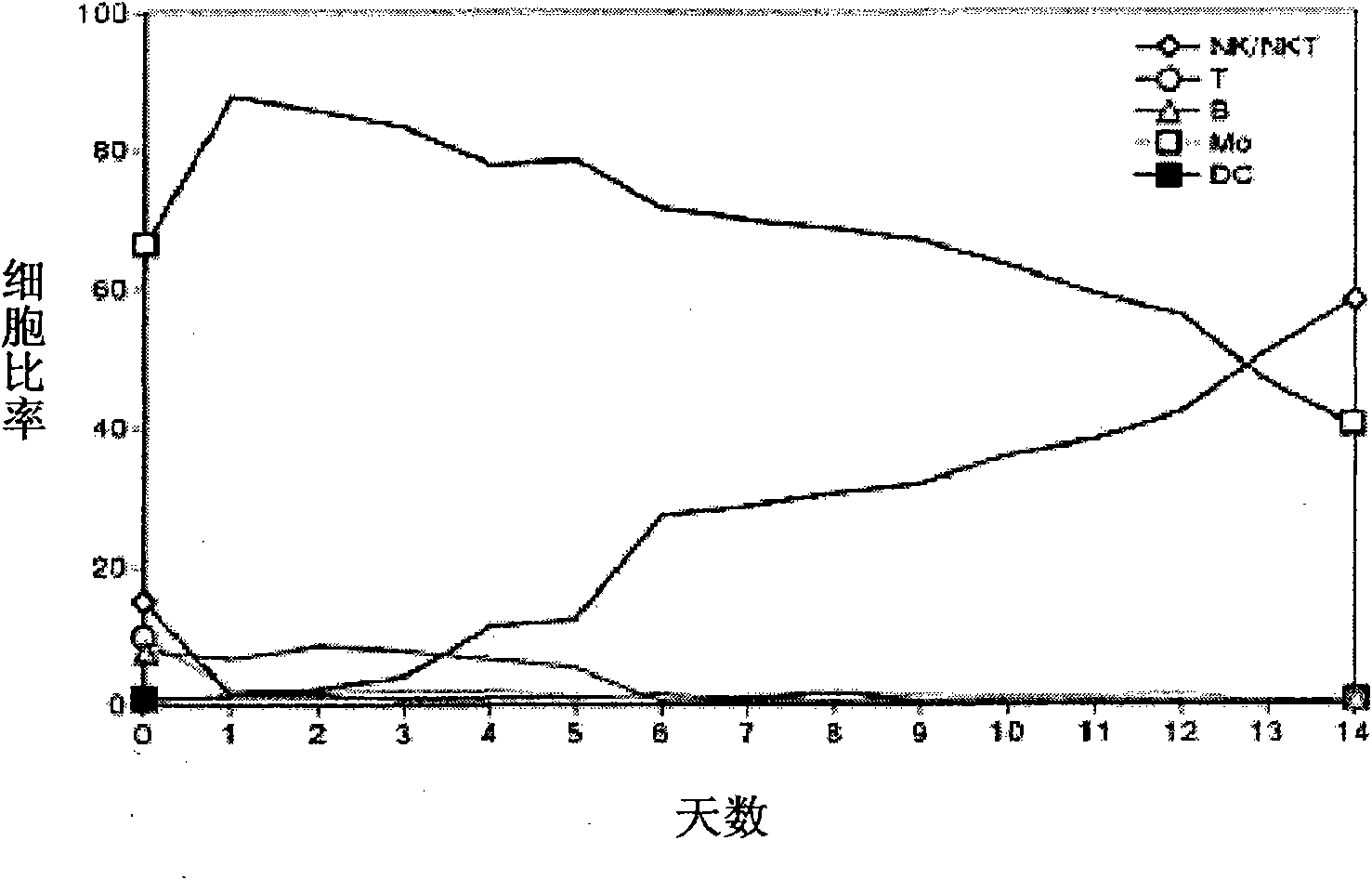
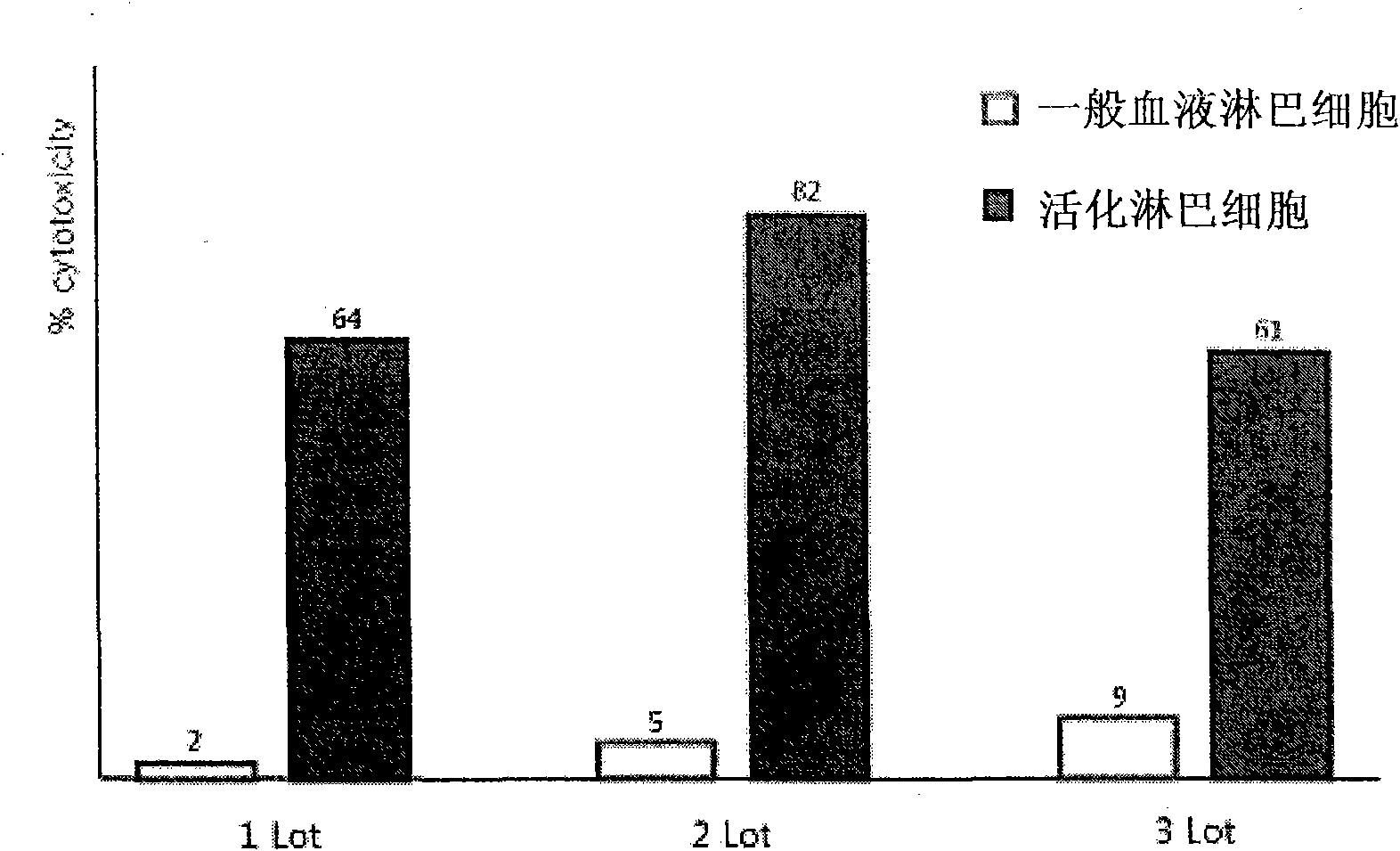
Provided is a method of culturing self-activated lymphocytes applicable to the treatment of malignant tumors. The method raises the percentage's of natural killer (NK) cells of the lymphocytes and evenly activate the NK cells, T cells and natural killer T (NKT) cells, and thus can be used to effectively eliminate various kinds of cancer cells. The method of culturing self- activated lymphocytes involves: extracting lymphocytes from human peripheral blood; performing a first culturing step of culturing the extracted lymphocytes in a culture fluid to which IL-2, L-glutamine and autochthonous plasma are added, in the presence of anti-CD3, anti- CD 16, and anti-CD56 antibodies; and performing a second culturing step of culturing the mixed culture fluid resulting from the first culturing step in the presence of anti-CD3, anti-CD16, and anti-CD56 antibodies after being admixed with a culture fluid to which IL-2, L-glutamine and autochthonous plasma are added.
Owner:NKBIO
Chinese medicine formulation for treating cancer and preparation method thereof
InactiveCN101401915AEase back to normalRelief and return to normal activityAntineoplastic agentsPlant ingredientsSide effectLymphatic Spread
The invention provides a Chinese medicine preparation for treating malignancy and a preparation method thereof. The preparation method comprises the following steps: firstly, pseudo-ginseng, Rhizoma Zedoariae, fritillariae thunbergii, Atractylodes macrocephala, edible tulip and Polyporus umbellate are dried and crushed into fine powder according to certain weight percent; secondly, Tephroseris kirilowii, Scutellaria barbata, Artemisia capillaries, radix astragali and liquorice with certain weight percent are added with water for decoction, a decoction solution is formed and filtered, and filtrate is decompressed and concentrated into condensed ointment; and thirdly, the fine powder and the condensed ointment are uniformly mixed, dried and crushed into fine powder which is then uniformly mixed and added with adequate water, so as to obtain the Chinese medicine preparation for treating the malignancy. The Chinese medicine preparation has obvious treatment effect on malignancy, particularly has obvious effects on preventing post-operation recurrence and metastasis and lightening the toxic side effect of chemicotherapy, is applicable to various cancer patients without untoward reaction and toxic side effect, relieves the symptoms and prolongs the life of the patients. The preparation method has simple technique, can improve the quality of the medicine and effectively improve the clinic treatment effect of the medicine.
Owner:西安国医肿瘤医院
Method for treating malignant tumors with joint medicament administration and anti-malignant tumor medicament
ActiveCN101669941AGrowth inhibitionLow effective doseOrganic active ingredientsAntineoplastic agentsTumor therapyWilms' tumor
The invention discloses a method for treating malignant tumors with joint medicament administration and also discloses an anti-malignant tumor medicinal composition with an antitumor activity and a low toxicity and an anti-malignant tumor medicinal preparation using the medicinal composition as an active component. The method comprises a step of jointing administrating anti-inflammatory medicaments, such as glucocorticosteroids or immunosuppressive agents or biotic agents of target cell factors and a carboxamidotriazole or derivatives or intermediates of the carboxamidotriazole as a medicamentto treat malignant tumors, wherein the anti-inflammatory medicaments have a certain regulation effect on cell factors. Tests prove that the combined medicament can increase the sensitivity of tumor cells to the carboxamidotriazole, so the combined medicament can be used as a broad-spectrum medicament for treating a plurality of malignant tumors, particularly non-small cell lung cancer, colon cancer and the like.
Owner:GUANGDONG YINZHU PHARMACEUTICAL TECHNOLOGY CO LTD
A medium composition for cultivating self activated lymphocyte
InactiveCN101603028AEnhance killing activityLittle side effectsCancer antigen ingredientsBlood/immune system cellsCD16Cell culture media
Provided is a medium composition for culturing self- activated lymphocytes applicable to the treatment of malignant tumors, to which anti-CD3, anti-CD16 and anti-CD56 antibodies are added along with interleukin2 (IL-2) to evenly activate natural killer (NK) cells, T cells and natural killer T (NKT) cells, and thus the medium composition can be used to culture im- munocytes that can effectively treat various kinds of malignant tumors. The medium composition includes a cell culture medium and additives added to the cell culture medium, wherein the additives include interleukin2 (IL-2), anti-CD3 antibodies, anti-CD16 antibodies, and anti-CD56 antibodies.
Owner:NKBIO
Antigenic polypeptide usable as therapeutic agent for malignant neoplasm
ActiveUS20100034841A1Promote productionTumor rejection antigen precursorsPeptide/protein ingredientsEpitopeNeoplasm
This invention provides a new tumor antigen having an epitope that induces a Th1 cell (a CD4-positive T cell specific to MAGE-A4), and a method of application thereof.Specifically, this invention provides a polypeptide that includes an amino acid sequence having the following characteristic a), b), c), d), e) or f) and has a cytokine-producing activity in a Th cell specific to MAGE-A4:a) An amino acid sequence represented by SEQ ID No: 1 or No: 2;b) An amino acid sequence, wherein one to several tens of any amino acids are added to N terminus and / or C terminus of an amino acid sequence represented by SEQ ID No: 1 or No: 2;c) An amino acid sequence, wherein one to five amino acids from N terminus and / or C terminus of an amino acid sequence represented by SEQ ID No: 1 or No: 2 are deleted;d) An amino acid sequence, wherein one to several amino acid residues of an amino acid sequence represented by SEQ ID No: 1 or No: 2 are substituted and / or deleted;e) An amino acid sequence, wherein one to several tens of any amino acids are added to N terminus and / or C terminus of an amino acid sequence, wherein one to several amino acid residues of an amino acid sequence represented by SEQ ID No: 1 or No: 2 are substituted and / or deleted; andf) An amino acid sequence, wherein one to several amino acid residues are substituted in an amino acid sequence, wherein one to five amino acids from N terminus and / or C terminus of an amino acid sequence represented by SEQ ID No: 1 or No: 2 are deleted.
Owner:HOKKAIDO UNIVERSITY
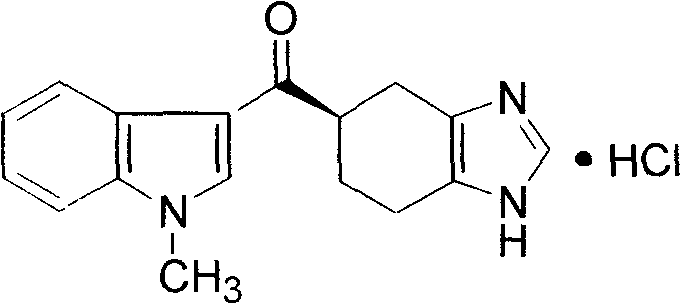
Ramosetron hydrochloride freeze-dried powder injection and preparation method thereof
ActiveCN103211771AEasy to shapeLoose appearancePowder deliveryOrganic active ingredientsTreatment effectFreeze-drying
The invention discloses a ramosetron hydrochloride freeze-dried powder injection and a preparation method thereof. Under illumination, the solution of the existing ramosetron hydrochloride has poor stability, poordrug effects and high drug adverse effects; and the solids of the existing ramosetron hydrochloride have good stability. Therefore, the ramosetron hydrochloride freeze-dried powder injection improves product stability and safety. A preclinical research shows that an antagonistic activity of ramosetron hydrochloride to 5-HT3 acceptors is stronger and more stable than that of the existing 5-HT3 acceptor antagonist; and ramosetron hydrochloride has stronger effect of treatment on chemotherapeutic drug-caused emesis. Therefore, ramosetron hydrochloride is clinically used for preventing and treating digestive tract symptoms such as nausea and emesis caused by cancer-resistant drugs.
Owner:ZHEJIANG YATAI PHARMA
Rheum emodin double-chain biquaternary ammonium salt with anti-cancer activity and preparation method of rheum emodin double-chain biquaternary ammonium salt
InactiveCN104311434AHigh anticancer activityEfficient killingOrganic compound preparationAntineoplastic agentsBenzoyl bromidePharmaceutical drug
The invention discloses a rheum emodin double-chain biquaternary ammonium salt with the anti-cancer activity and a preparation method of the rheum emodin double-chain biquaternary ammonium salt. The rheum emodin double-chain biquaternary ammonium salt is a mixture of a rheum emodin 1,3-site biquaternary ammonium salt and a rheum emodin 3,8-site biquaternary ammonium salt. The preparation method comprises the following steps: performing Williamson etherification reaction on rheum emodin and p-benzyl bromide in the presence of K2CO3 so as to generate a mixture of 1,3-site dibromomethylbenzyl rheum emodin and 3,8-site dibromomethylbenzyl rheum emodin, and further reacting the dibromomethylbenzyl rheum emodin mixture with tertiary amine so as to obtain the mixture of 1,3-site biquaternary ammonium salt and rheum emodin 3,8-site biquaternary ammonium salt, wherein the two biquaternary ammonium salts are isomerides which cannot be separated on a chromatographic column. The anti-cancer activity evaluation shows that the activity of the rheum emodin double-chain biquaternary ammonium salt disclosed by the invention is higher than that of monoquaternary ammonium salt, and the rheum emodin double-chain biquaternary ammonium salt can be used in a medicine for treating malignant tumor, is particularly applicable to treatment on liver cancer, has a relatively small inhibition effect on normal cells, and has relatively great application prospect.
Owner:FUZHOU UNIV
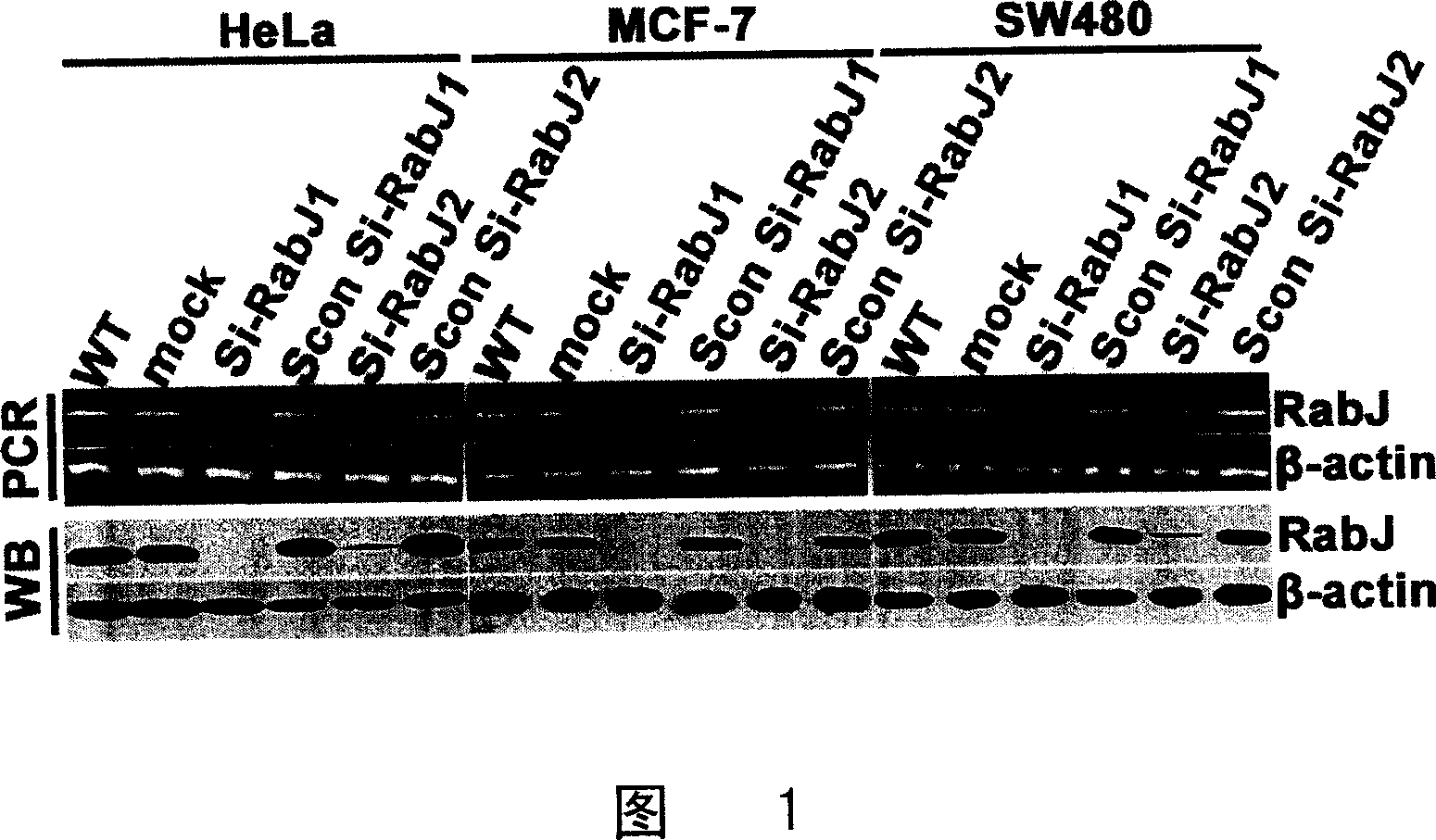
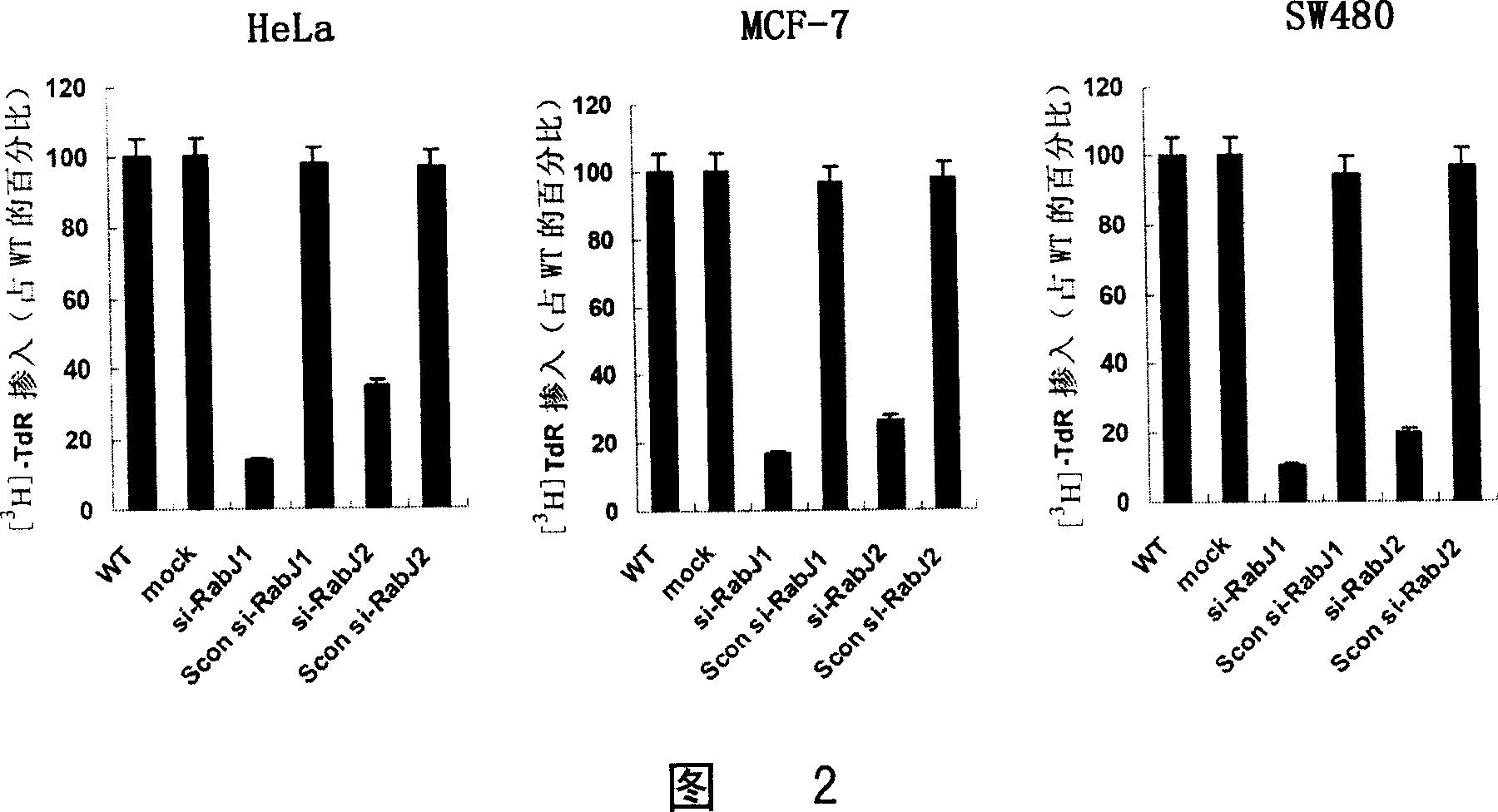
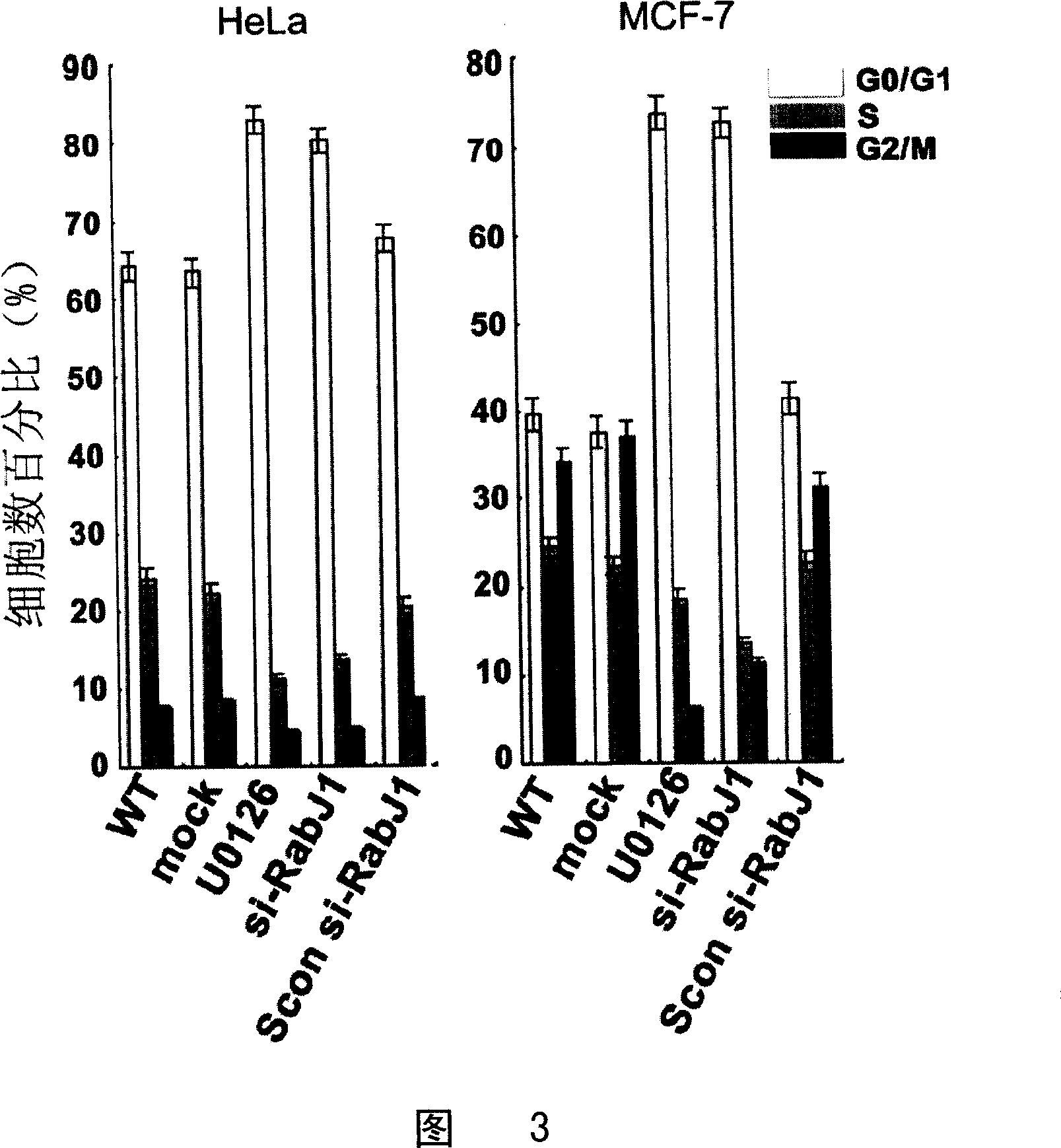
SiRNA for inhibiting human Rabj gene expression and its application
This invention relates to an interfering RNA nucleotide sequence aiming directly at oncogene-like molecule RabJ. Exactly to say, this invention relates to a interfering RNA nucleotide sequence which aims directly at the expression and function of oncogene-like molecule RabJ, the interfering RNA nucleotide sequence possesses gene expression and function of target anti- tumour cell RabJ, having the function of restraining tumour cell to grow and oncogenicity of tumour cell, and having the function of promoting apoptosis, thus contributing to cure tumor. This invention also relates to medicine Combination containing this interfering RNA nucleotide sequence, and discloses the method of using this medicine of interfering RNA for disease therapy, especially it is used to cure hyper-expressed malignant tumor.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Medium composition for culturing self-activated lymphocytes and method for culturing self-activated lymphocytes using same
InactiveCN103080302ARaise the ratioLittle side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer antigen ingredientsCD16Interleukin II
Disclosed is a medium composition for culturing self-activated lymphocytes, which contains anti-CD3 antibody and anti-CD16 antibody in addition to interleukin 2 (IL-2), interleukin 12 (IL-12) and interleukin 18 (IL-18) in a medium, and thus can efficiently proliferate and activate NK cells, T cells and NKT cells and, at the same time, can significantly increase the ratio of NK cells in lymphocytes so as to provide immunocytes having excellent effects on the treatment of various kinds of malignant tumors, and a method for culturing self-activated lymphocytes using the medium composition.
Owner:CELLS SCI CORP
Therapeutic vaccine for malignant tumors and composition thereof
The invention relates to a therapeutic vaccine for malignant tumors and a composition thereof. The therapeutic vaccine for malignant tumors is a tumor cell line which contains plasmid of antisense nucleic acid of human transforming growth factor beta (TGF-beta2); the therapeutic vaccine composition for malignant tumors comprises the therapeutic vaccine for malignant tumors and an immunopotentiator, and the immunopotentiator is one selected from the group consisting of a Corynebacterium parvum preparation, a non-cell Corynebacterium parvum preparation, a BCG polysaccharide, a nucleic acid preparation, a Nocardia rubra-cell wall skeleton preparation, a group A Streptococcus preparation, a non-cell group A Streptococcus preparation, a Pseudomonas aeruginosa preparation, a non-cell Pseudomonas aeruginosa preparation, a Brucella preparation, a non-cell Brucella preparation, a non-cell Mycobacterium vaccae preparation and a non-cell Mycobacterium smegmatis preparation, preferably the non-cell Corynebacterium parvum preparation; and the malignant tumors include a lung cancer, a liver cancer, a pancreatic cancer, leukemia, lymphoma, an ovarian cancer, a colon cancer, a stomach cancer and a breast cancer.
Owner:熊慧
Dominant sequence of delta 1 chain complementary determining region (CDR) 3 in gamma delta T lymphocytes, and T cell receptor (TCR) transfected cells and application thereof
The invention discloses a dominant sequence GTM of a delta 1 chain complementary determining region (CDR) 3 in human gastric cancer tissue-derived tumor-infiltrating gamma delta T lymphocytes, a T cell receptor (TCR) containing the sequence, and TCR transfected cells. The amino acid residue sequence of the GTM is shown as SEQ ID NO.1 in a sequence table. Experiments prove that GTM peptide can be specifically combined with tumor cells, tumor tissue and V delta 1 T cell ligand MICA protein; and a J.RT3-T3.5 cell platform for expressing the TCR containing the dominant sequence GTM of the delta 1 chain CDR3 in the human gastric cancer tissue-derived tumor-infiltrating gamma delta T lymphocytes on a J.RT3-T3.5 surface is established by a lentivirus expression system, gamma delta 1 tumor-infiltrating lymphocytes (TIL) and a tumor identifying and killing mechanism thereof are simulated in the cell level, the killing activity of the transfected cells TCR gamma 4 delta 1 on the tumor cells is obviously increased, and the killing effect is TCR-dependent. The invention plays an important role in the development of medicines for treating malignant tumors and the adoptive treatment of malignant tumors, and has wide application prospects.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
An antineoplastic dendritic polymer drug delivery system
InactiveCN1558777AImprove anti-tumor activityImprove performanceHeavy metal active ingredientsPharmaceutical non-active ingredientsSolubilityDrug release
Antineoplastic dendritic polymer conjugates which are useful drug delivery systems for carrying antineoplastic agents to malignant tumors are prepared. The antineoplastic agent is encapsulated within the dendritic polymer using an ionic charge shunt mechanism, whereby, the antineoplastic agent interacts with the anionic functional groups on the surface of the dendritic polymer allowing the antineoplastic agent to be uptaken by the dendritic polymer through an association with the functional groups of the interior of the dendritic polymer. The antineoplastic dendritic polymer conjugates may be administered intravenously, orally, parentally, subcutaneously, intraarterially or topically to an animal having a malignant tumor in an amount which is effective to inhibit growth of the malignant tumor. The antineoplastic dendritic polymer conjugates exhibit high drug efficiency, high drug carrying capacity, good water solubility, good stability on storage, and reduced toxicity.
Owner:DENDRITIC NANO TECH INC
Method for producing monoclonal antibodies by using transgenic animal mammary gland bioreactor
InactiveCN104862318AHigh purityEasy to separateMilk preparationImmunoglobulins against animals/humansDiseaseAutoimmune disease
The present invention relates to a method for large-scale production of recombinant monoclonal antibodies by using a transgenic animal mammary gland biological platform, and specifically provides an antibody element construct or expression cassette for specific expression of the mammary gland, wherein nuclear transplantation is performed after the construct or expression cassette is transformed into mammalian cells so as to make the transgenic animal mammary gland express and secrete the exogenous recombinant antibody protein, such that the monoclonal antibodies with characteristics of easy separation and easy purification are prepared. According to the present invention, the method is simple and efficient, and the produced recombinant monoclonal antibodies can be widely used in diagnosis, prevention, and treatment of cancers and other serious diseases research, and immune mechanism research, especially in the field of malignant tumor treatment or autoimmune disease treatment.
Owner:NANJING JENOMED BIOTECH CO LTD
Preparation method of ramosetron derivatives and applications thereof
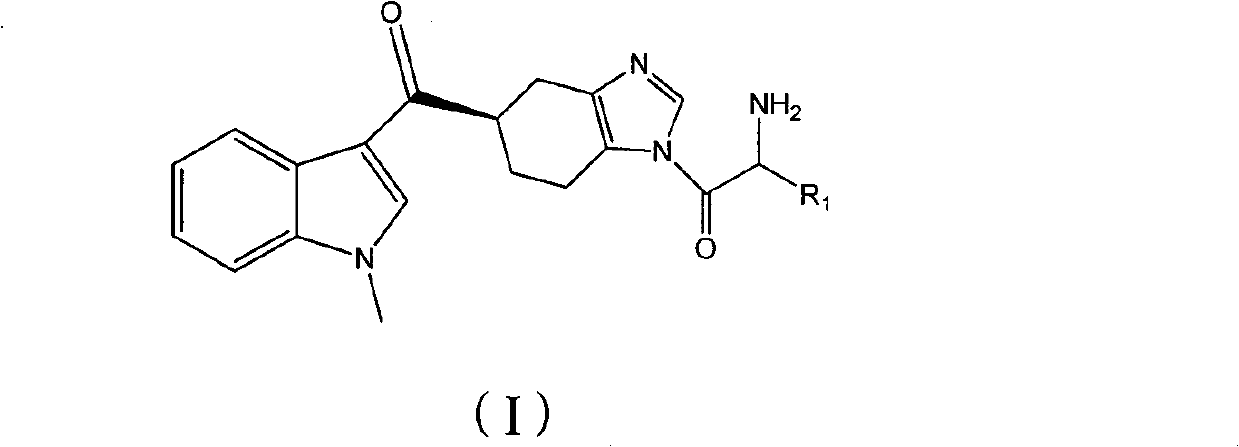
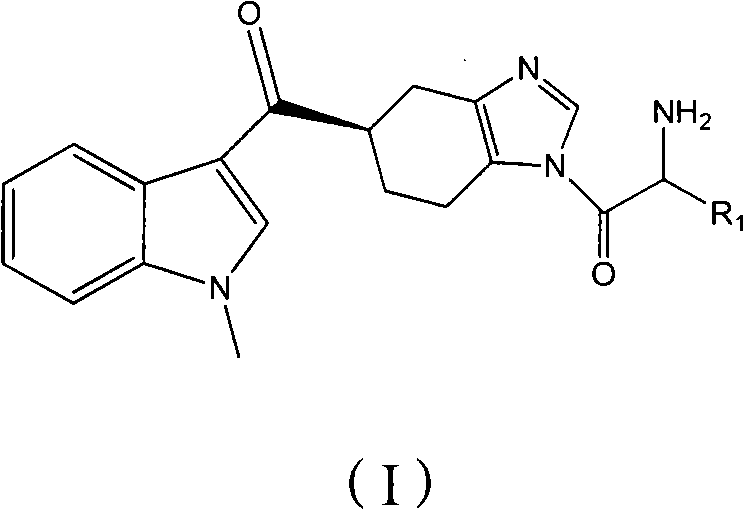
ActiveCN102101858AGood water solubilityEnhance pharmacological effectsOrganic active ingredientsOrganic chemistryMalignant Neoplasm TreatmentIrritable bowel syndrome
The invention relates to a preparation method of ramosetron derivatives and applications thereof. The invention provides a compound as expressed in the formula (I), a pharmaceutically acceptable salt thereof, an enantiomer or racemic mixture thereof, wherein, R1 is H, C1-C6 alkyl, C1-4 alkyloxys, aryls or substituted C1-C4 alkyl. The invention also provides a preparation method of the compound or the pharmaceutically acceptable salt thereof, the pharmaceutical composition containing the compound and the salt, and applications of the compound. The compound in the invention possesses functions of preventing and treating the digestive tract symptoms of nausea, vomit, etc. caused by anti-malignant tumor treatment, and preventing and treating irritable bowel syndrome. The compound can be used in preparing medicine for preventing and treating the digestive tract symptoms of nausea, vomit, etc. caused by anti-malignant tumor treatment, and irritable bowel syndrome.
Owner:天津康鸿医药科技发展有限公司
Anti-tumor tree-shaped polypeptide macromolecule medicament carrier system
InactiveCN101461946AGrowth inhibitionImprove performancePharmaceutical non-active ingredientsAntineoplastic agentsSolubilityCarrier system
The invention relates to an anti-tumor dendric polypeptide macromolecule medicine vector system and application thereof in pharmacy. The dendric polypeptide macromolecule can be prepared by the solid phase, liquid phase or solid-liquid combination method of single or different natural amino acids, and can be used for preparing a vector release system which is loaded with malignant tumor resisting treatment medicines. The dendric polypeptide macromolecule medicine vector system is a covalent bond vector medicine system in which anti-tumor activity micromolecule medicines are sealed in dendric polypeptide macromolecule polymers, or the anti-tumor activity micromolecules are combined with functional groups on the surfaces of the anti-tumor dendric polypeptide macromolecules. The dendric polypeptide macromolecule medicine vector system can correspondingly inhibit growth of the malignant tumor and is applied to a patient suffered from the malignant tumor in the modes of venous, oral-taking, endermic and arterial administration or local administration. The anti-tumor dendric polypeptide macromolecule medicine vector system has the advantages of high targeting property, high medicine carrying capability, good waster solubility and stability and lower toxicity.
Owner:刘湖
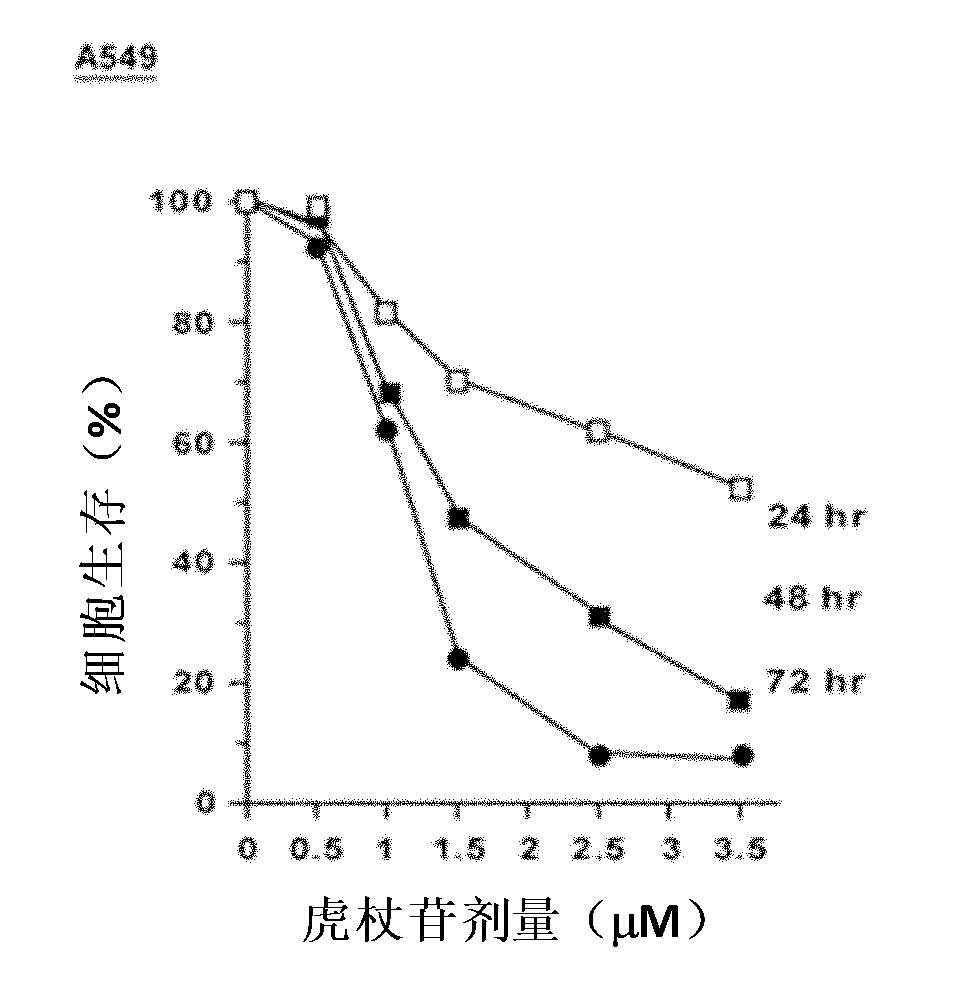
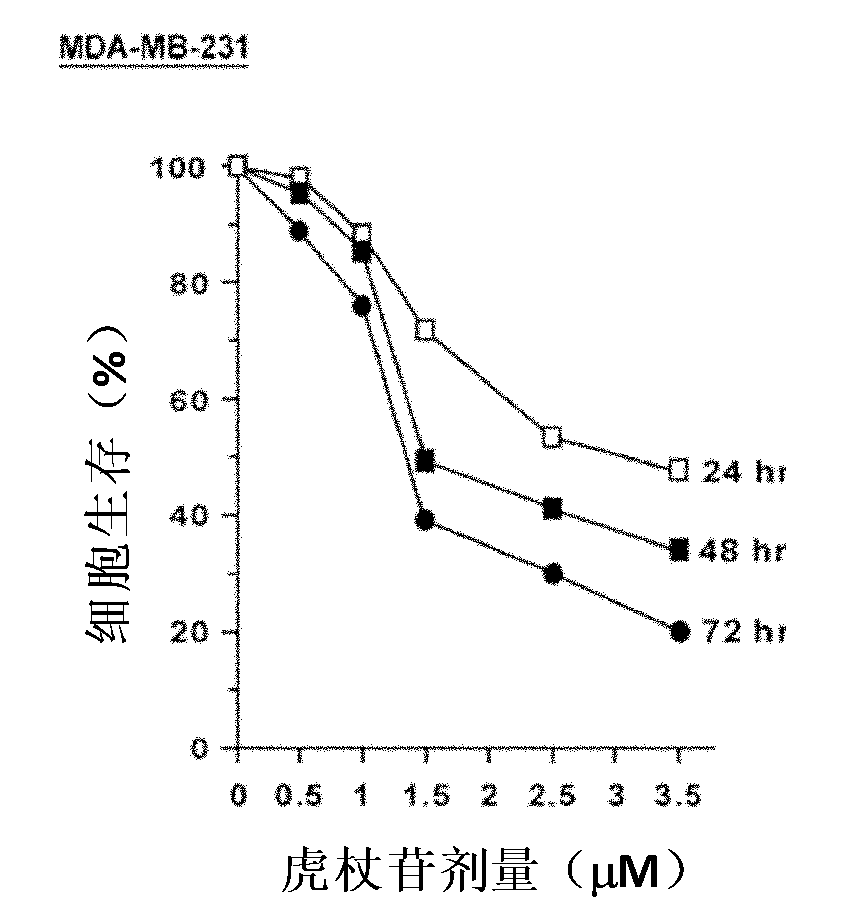
Application of polydatin to preparing antineoplastic drug
InactiveCN102058609APrevent proliferationInhibit migrationOrganic active ingredientsAntineoplastic agentsSide effectLife quality
The invention belongs to the field of antineoplastic drug preparation and relates to novel application of polydatin to preparing the antineoplastic drugs. The invention also provides an antineoplastic drug, wherein the main active component of the antineoplastic drug is polydatin. In the invention, the proliferations of cells such as breast cancer, lung cancer, liver cancer, ovarian cancer, cervical cancer, nasopharyngeal cancer, leukemia and the like treated by the antineoplastic drug polydatin are all restrained obviously; a cell cycle analysis shows that the S phase in the cell cycle is retardant and cell apoptosis is induced; and the polydatin with low concentration has the effect of restraining the migration and the conglutination of a tumor cell. In the invention, while the antineoplastic drug polydatin increases the treatment effect of a malignant tumor and reduces the incidence rate of relapse and transfer of a tumor patient, the toxic and side effects are obviously reduced compared with the traditional clinical commonly used chemotherapeutic drugs, therefore, the lifetime of a malignant tumor patient is prolonged, and the life quality of the malignant tumor patient is improved.
Owner:SUZHOU UNIV
Malignant tumor therapeutic vaccine and composition thereof
InactiveCN104096238AStrong immune antigen specificityLow priceGenetic material ingredientsAntibody medical ingredientsCancer cellHuman tumor
The invention relates to a malignant tumor therapeutic vaccine and a composition thereof. The malignant tumor therapeutic vaccine is a tumor cell line obtained by culture of a human tumor tissue's cancer cells. A TGF-beta2 antisense plasmid is transfected into the tumor cell line, and the tumor cell line is subjected to inactivation, thus obtaining the malignant tumor therapeutic vaccine. The invention also relates to a malignant tumor therapeutic vaccine composition, which includes the malignant tumor therapeutic vaccine and an immunopotentiator. The malignant tumor therapeutic vaccine provided by the invention can be used for treating the tumor cell sourced patients, and has the advantages of strong immune antigen specificity, repeated preparation and use and low price, and also can be prepared into a vaccine preparation for treatment of other patients suffering a same cancer. Animal experiments confirm that the malignant tumor therapeutic vaccine has a good treatment effect.
Owner:熊慧
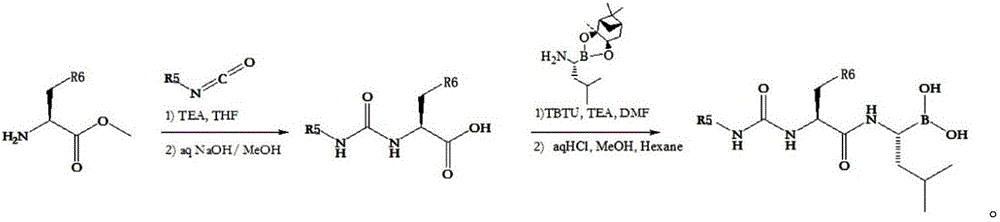
Class I dipeptidyl boric acid compound and preparation method and application thereof
ActiveCN106008572ANovel structureBlock proliferationGroup 3/13 element organic compoundsAntineoplastic agentsDiseaseChemical compound
The invention discloses a class I dipeptidyl boric acid compound and a preparation method and an application thereof, and aims to provide a novel boric acid compound with a novel structure and a function of restraining proteasome. The class I dipeptidyl boric acid compound is shown as a formula I shown in the description, wherein R is shown in the description. The class I dipeptidyl boric acid compound has the effects of blocking tumor cell proliferation and inducing cancer cell apoptosis, so that the compound can be used for treating a plurality of diseases such as malignant tumors.
Owner:成都四面体药物研究有限公司
Preparation method for probiotics system and application of probiotics system
ActiveCN112972503ABroad tumor growth inhibitory effectGood effectDigestive systemInorganic non-active ingredientsIntestinal microorganismsLow Grade Malignant Neoplasm
Owner:NANJING UNIV
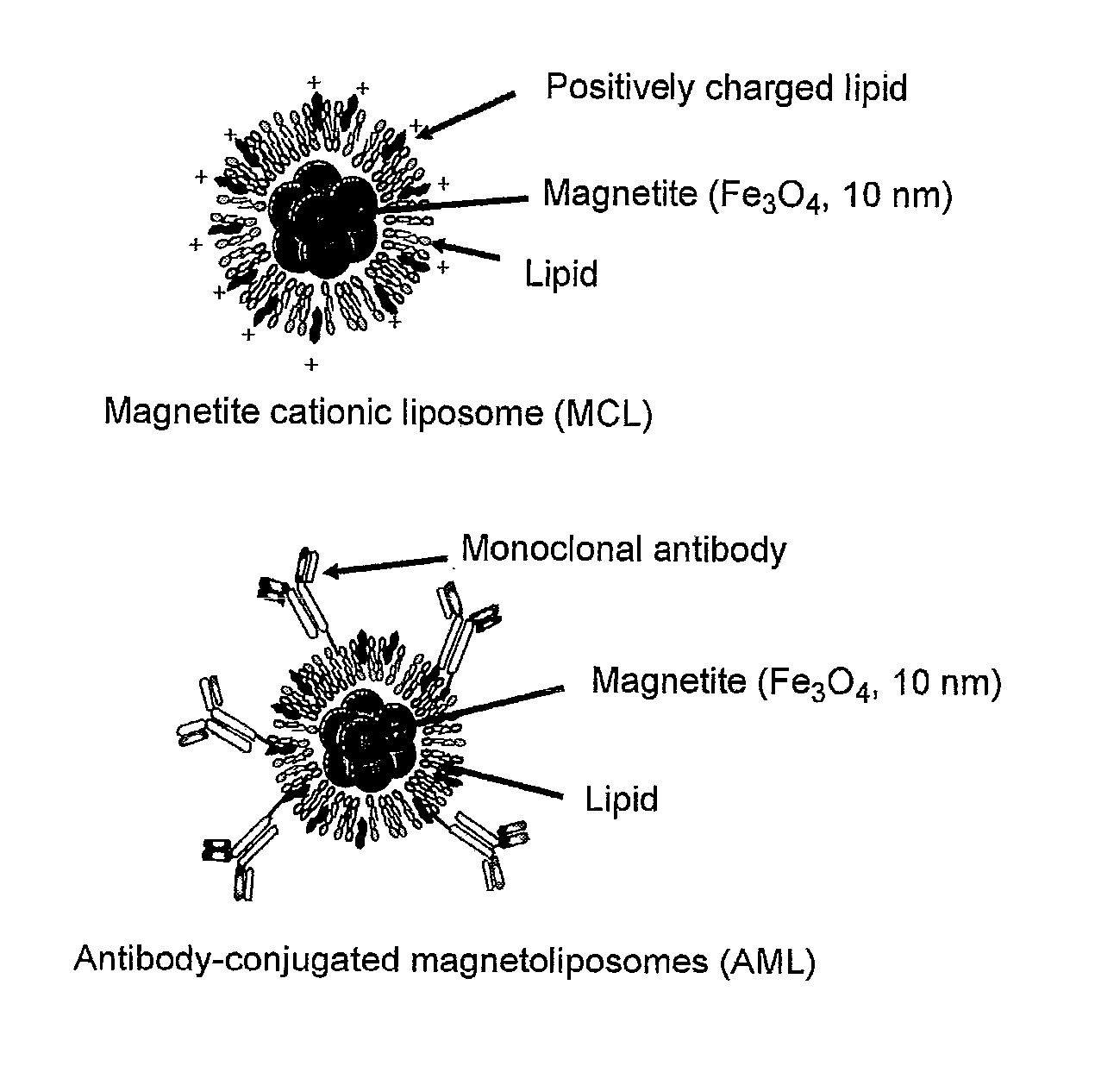
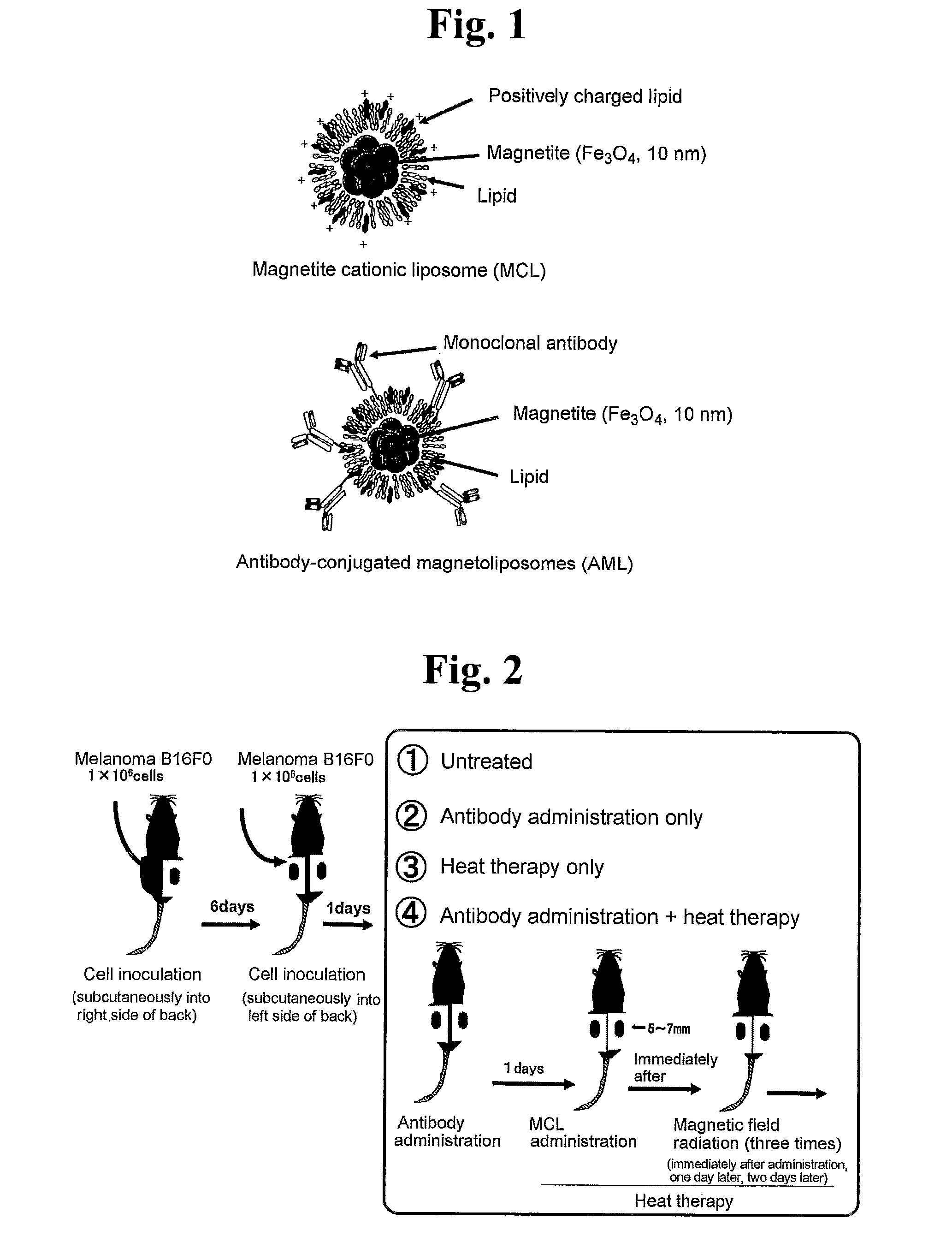
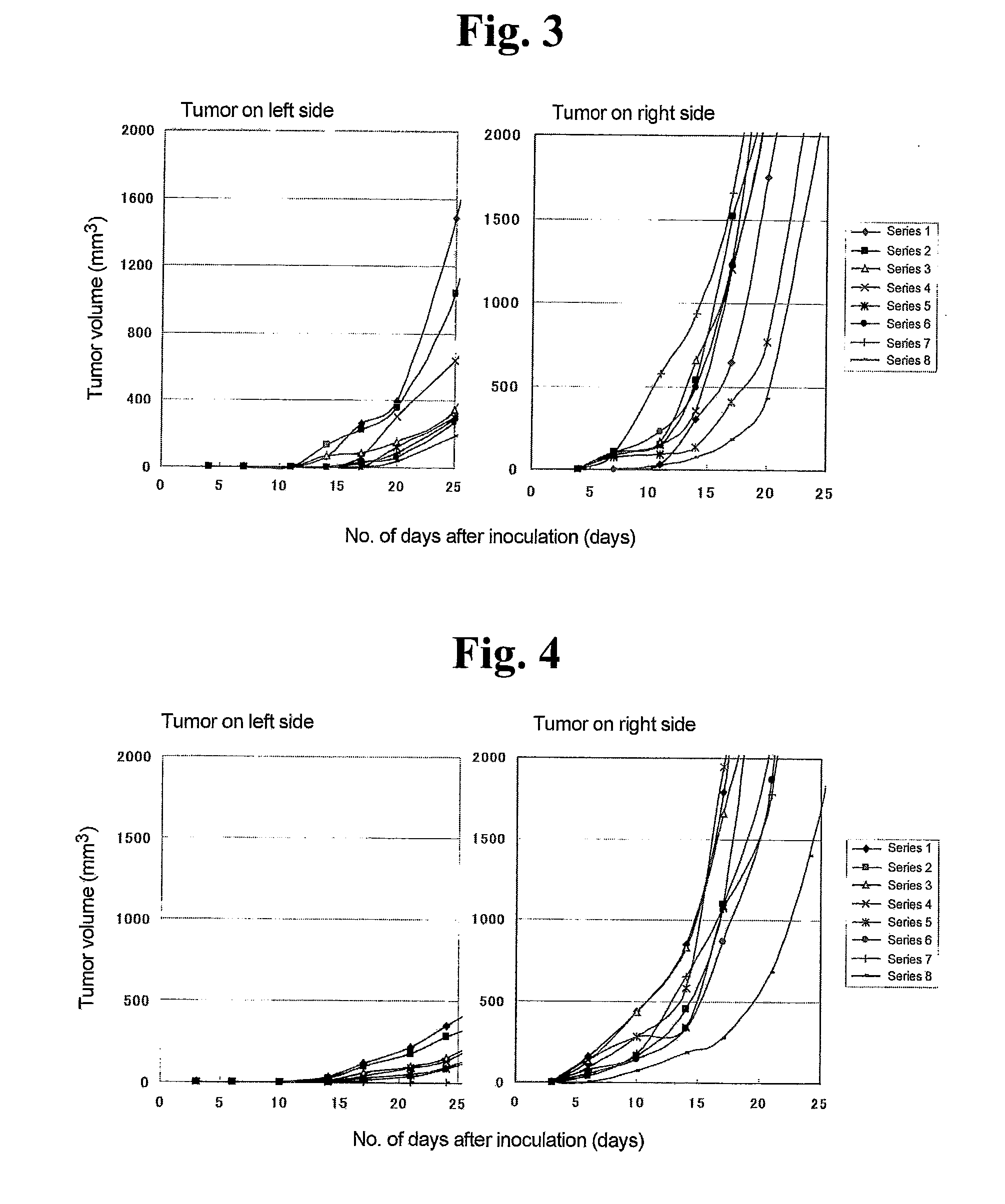
Malignant tumor heat therapy kit comprising Anti-regulatory t cell antibody and magnetic fine particles and heat therapy method thereof
InactiveUS20110165255A1Enhancing systemic antitumor immunityImprove immunityPowder deliveryHeavy metal active ingredientsRegulatory T cellMicroparticle
The present invention discloses a heat therapy kit for malignant tumor treatment that comprises a pharmaceutical agent containing anti-regulatory T cell antibody and a pharmaceutical agent containing magnetic fine particles, and a heat therapy method that uses that kit.
Owner:KOBAYASHI TAKESHI +1
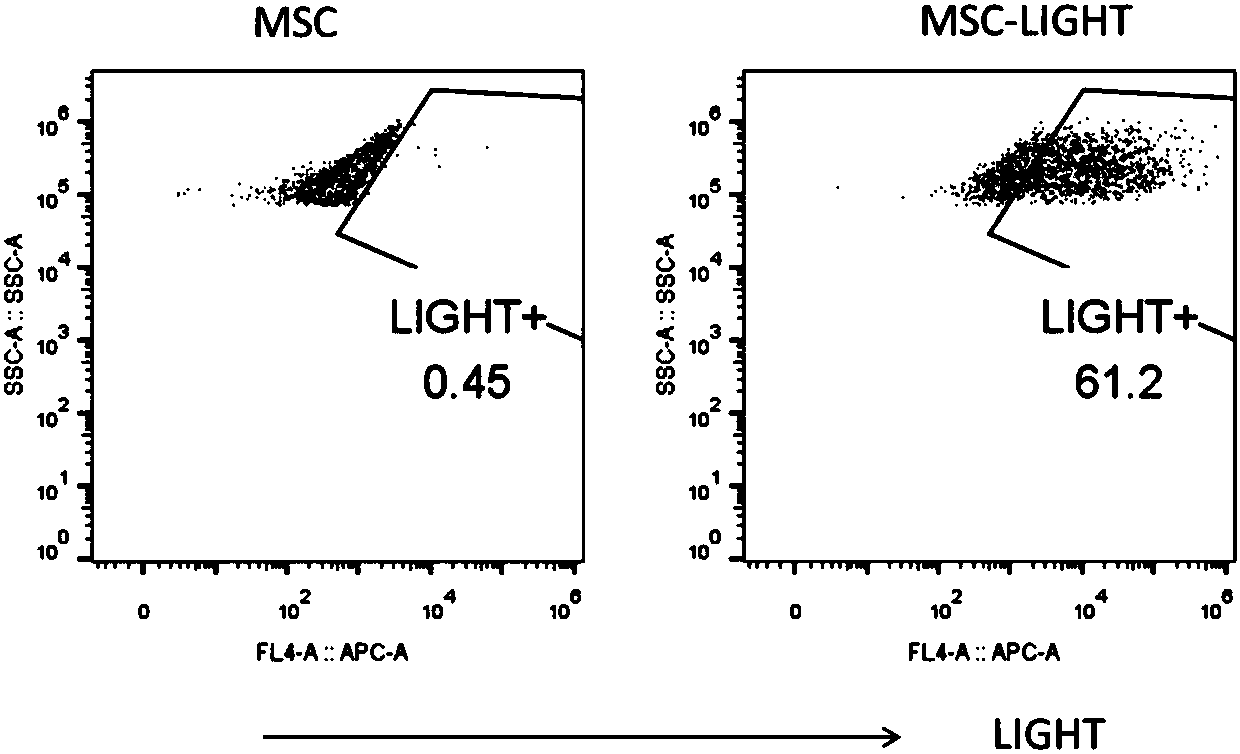
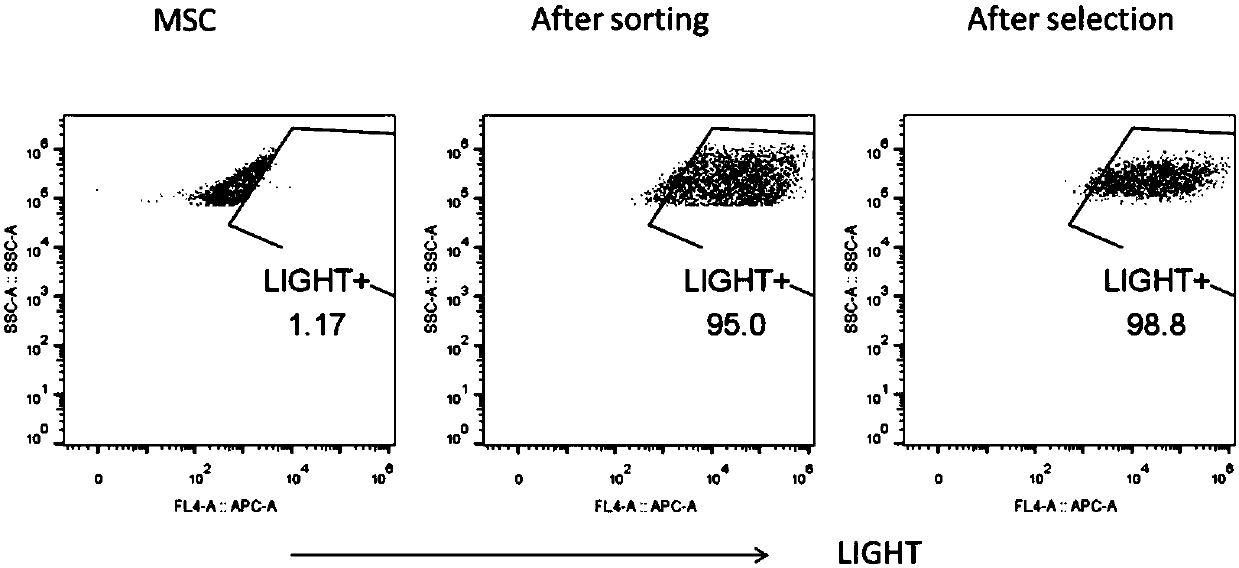
Preparation method of mesenchymal stem cell expressing human-derived immune stimulating factor LIGHT and prepared MSC-L cell
PendingCN107916253AEasy to collectReduced Chances of ContaminationGenetically modified cellsSkeletal/connective tissue cellsAbnormal tissue growthMesenchymal stem cell
The invention discloses a preparation method of a mesenchymal stem cell expressing a human-derived immune stimulating factor LIGHT. The preparation method comprises the following steps that the mesenchymal stem cell is separated and subjected to primary culture and secondary culture; and by building up a retroviral and lentiviral vector pMIGR3-hLIGHT and a pCDHEF-hLIGHT plasmid, a human LIGHT geneis transferred into the mesenchymal stem cell to build up an MSC-L cell, and the MSC-L cell is the mesenchymal stem cell expressing the human-derived immune stimulating factor LIGHT. The invention further relates to the MSC-L cell prepared by adopting the method and application of the MSC-L cell. According to the application of the MSC-L cell, an umbilical cord is adopted as an MSC source, the human LIGHT gene is transferred into the MSC, the LIGHT is transferred into a tumor tissue through the MSC, the MSC modified by the LIGHT can change the tolerance state of a host to a tumor by generating strong antitumor cells and immune reaction of tumor-related mesenchymal cells, and accordingly the tumor is controlled effectively. The mesenchymal stem cell can be prepared on a large scale, and used for treatment of various solid malignant tumors and prevention of cancers.
Owner:上海金坤生物科技有限公司
Drug-loaded stent with ultrasonic intelligent controlled release
InactiveCN102319453AWith ultrasonic intelligent controlled releaseConfirm new roleSurgeryCoatingsHuman bodyAnticarcinogenic Effect
The invention relates to a stent coating with ultrasonic intelligent controlled release and a preparation method and an application thereof. The invention also provides a drug-loaded stent with ultrasonic intelligent controlled release and a preparation method and an application thereof. The advantages of the invention are that: a new stent coating with the effect of ultrasonic intelligent controlled release is successfully constructed and synthesized; ultrasound is first found and confirmed to have control effect on the temperature-sensitive release of loaded drugs; and a new method is provided for future drug intelligent controlled release; a drug-loaded biliary tract stent with ultrasonic intelligent drug controlled release is synthesized, and a possible and feasible treatment means is provided for the treatment of malignant tumors of hollow organs; ultrasound is first found and confirmed to have significant effect on promoting the release of drugs coated by a temperature-sensitive material by changing the spatial structure of the temperature-sensitive material. By using ultrasound, the effect of drug intelligent controlled release at a designated site is achieved; local anticancer effect is brought into play; the toxic and side effect of anticancer drugs on important human tissue is reduced; and high clinical application value is provided.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com