An antineoplastic dendritic polymer drug delivery system
A dendritic and polymer technology, applied in antineoplastic drugs, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of the antitumor dendritic polymer complex is to dissolve the dendritic polymer in a suitable solvent, such as water, and the dendritic polymer and the dissolved antitumor agent are sufficient to make the two combine to form the dendritic polymer-anti-tumor agent. exposure to tumor agent complexes under conditions. The molar ratio of cisplatin to dendrimer (PAMAM with a degree of polymerization of 3.5, EDA core, and dendrimer dendrimer) is 35:1, which is the ratio used in the experiments in the examples of the present invention. The ratio of cisplatin to polymer molecules is important. Platinum esters of dendrimers having a molar ratio of cisplatin to polymer of 100:1 to 1:1 may have good practical advantages. Antineoplastic agents are encapsulated in polymers using shunt technology, and the anions (such as carboxylate groups) on the surface of the polymers have weak forces to enable antineoplastic agents to be taken up by dendrimers (primary act...
Embodiment 1
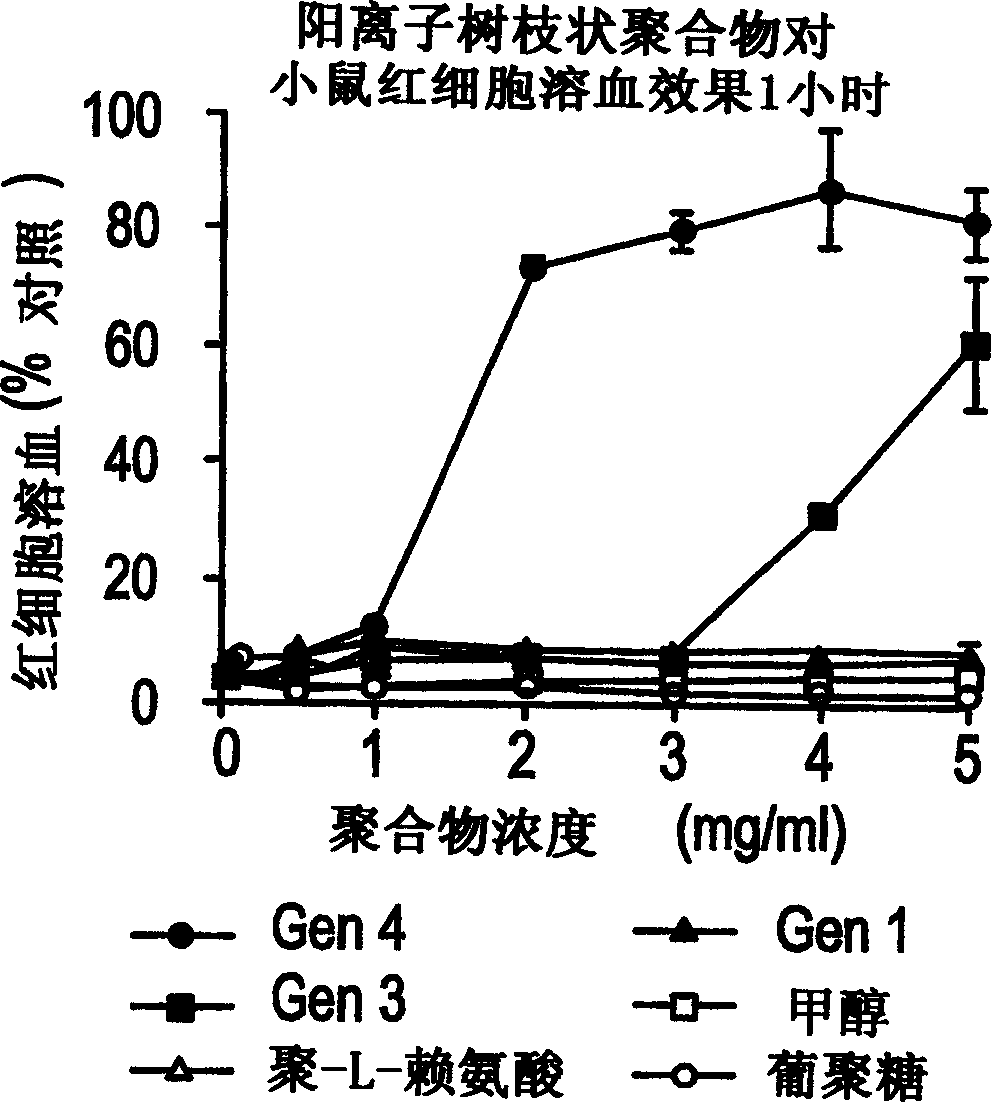
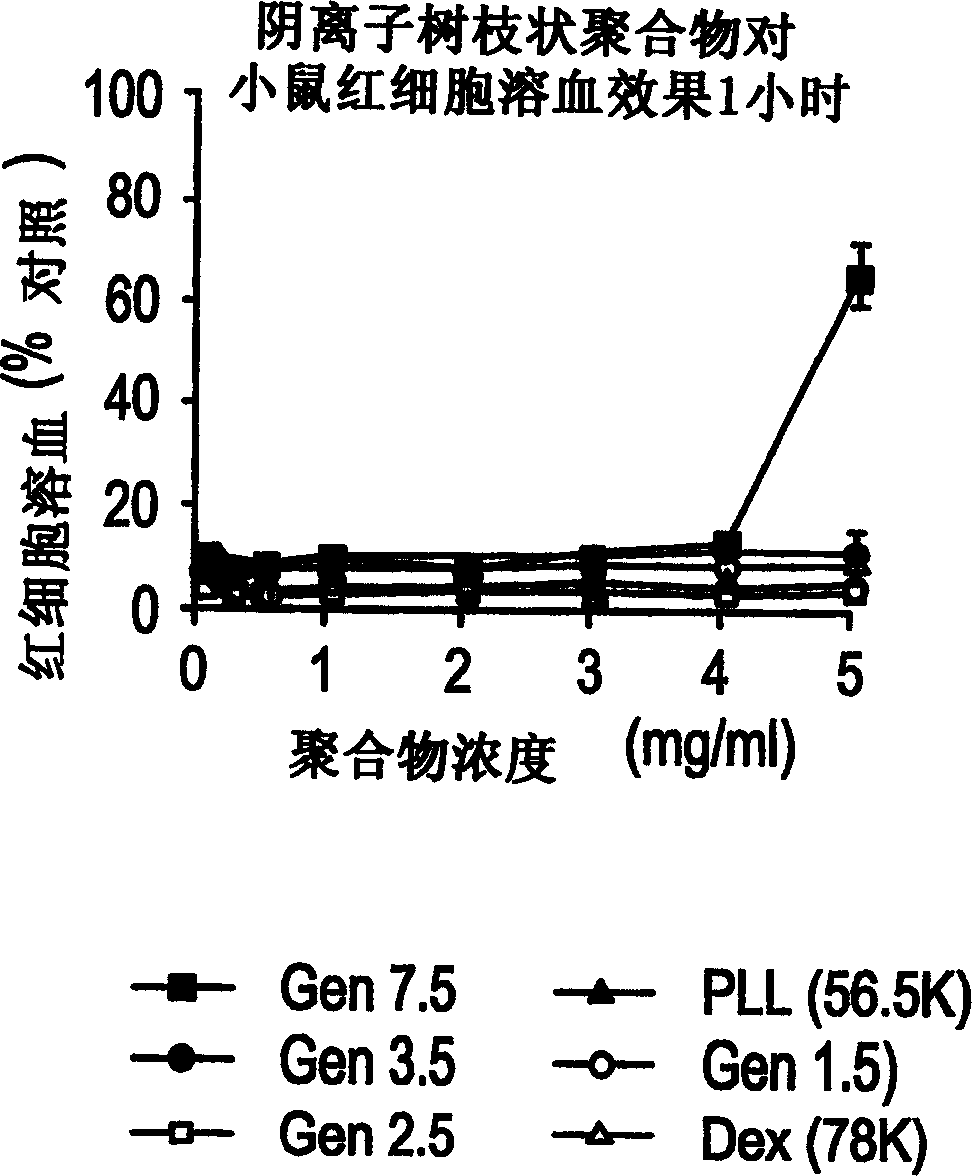
[0094] Example 1 Effects of PAMAM dendrimers on the stability of mouse erythrocytes cultured in vitro
[0095] method
[0096] Poly(amidoamine) dendrimers (cationic and anionic) of increasing degrees of polymerization were incubated with erythrocytes from adult Wistar rats. Interaction of dendrimer with erythrocytes was assessed spectrophotometrically by cytolysis-induced release of hemoglobin, detection at 550 nm. Different concentrations of dendrimer, control (methanol (BOH)), poly-L-lysine (hydrobromide-56.5KD Mw (Sigma)) and dextran (74KD Mw (Sigma)) (solution in physiological buffered saline) and mouse erythrocytes were co-cultured for 1 hour at 37°C and 10 rpm (in a shaking water bath). Afterwards, the erythrocytes were centrifuged at 1500×g for 10 minutes to pellet the cells, 100 μl of floating matter on the surface were removed, and PBS was used as a blank for spectrophotometric analysis. see attached results figure 1 and 2 As indicated, the percent hemoglob...
Embodiment 2
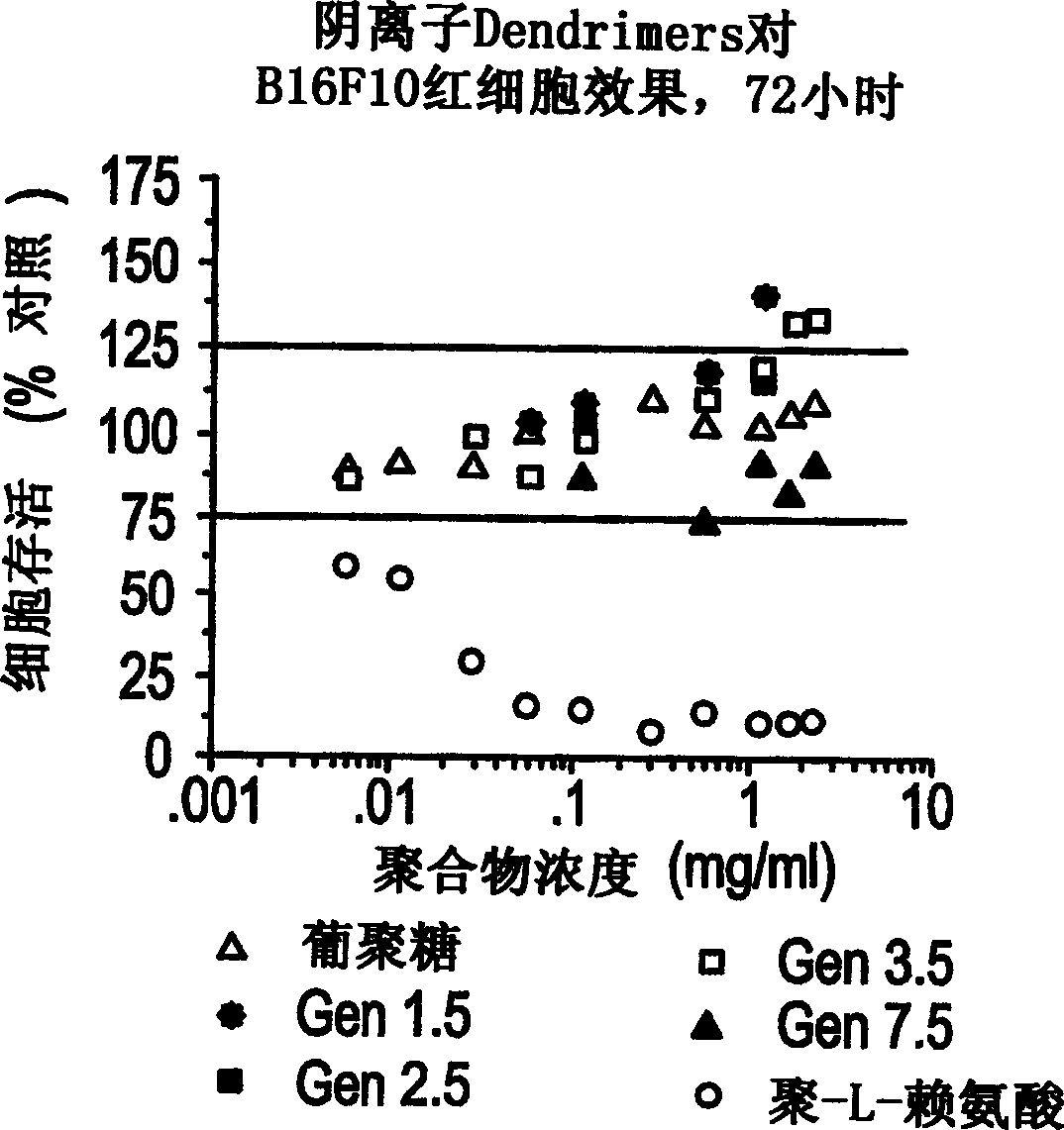
[0099] Example 2 Cytotoxicity of unmodified dendrimers on B16F10 cells
[0100] method
[0101] B16F10 cells are an adherent murine melanoma cell line. B16F10 cells at 1 x 10 per ml 5 pcs (1×10 per hole 4 cells) in 96-well microtiter plates (Costar) in RPMI 1640 tissue culture medium (Gibco) supplemented with 10% FCS (Gibco). All cell growth and cytotoxic incubations were performed at 37°C, 5% CO 2 under conditions.
[0102] Evaluation of cell density was performed with a modified neurenbrow cell counter (Sigma). Cells were washed twice with PBS and fresh RPMI medium (and supplemented with FCS) was added, and cells were then cultured on microtiter plates. Cells were left for 24 hours and recovered and reattached.
[0103] All polymers and controls were dissolved in RPMI medium (supplemented with FCS) and sterilized through a 0.2 [mu]m sterile filter with initial minimal loss of solution due to filter adhesion. Cells were then added at increasing concentrations of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com