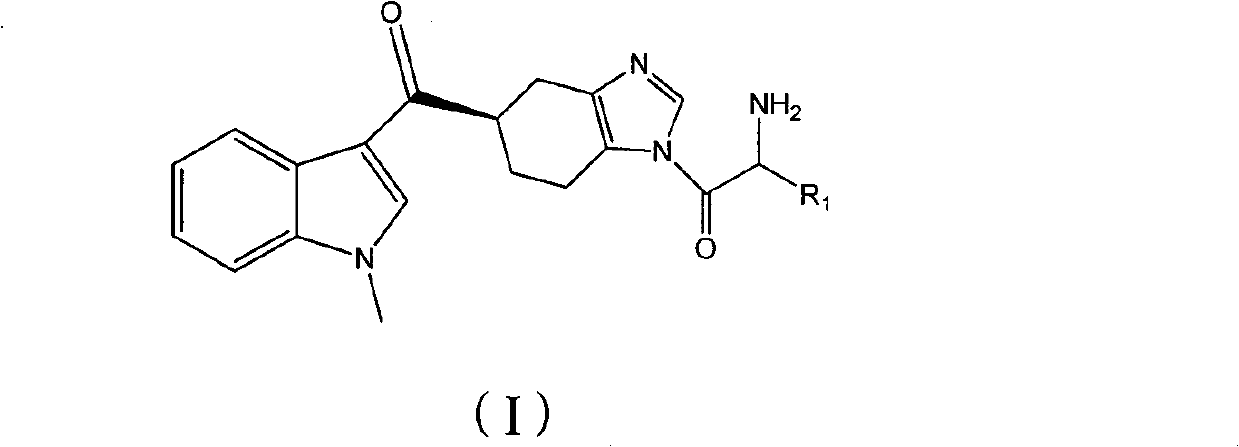
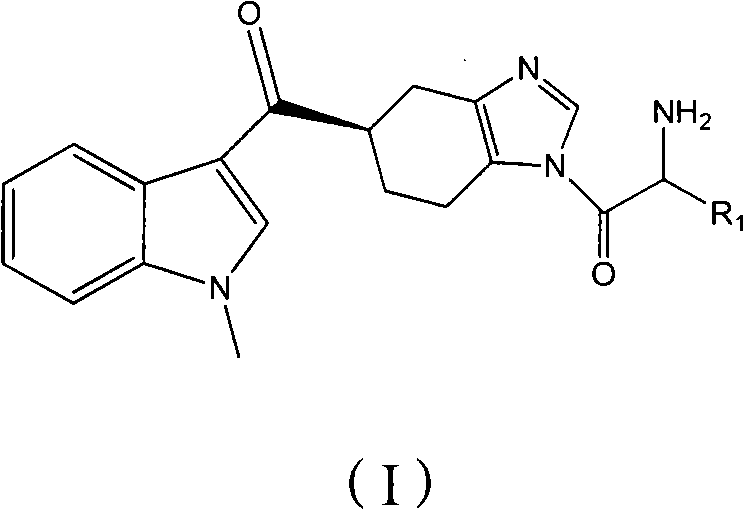
Preparation method of ramosetron derivatives and applications thereof
A technology for drugs and compounds, applied in the field of medicine, can solve the problems of water solubility and unsatisfactory bioavailability of alkanoyl ramosetron compounds, and achieve the effects of good pharmacological activity, easy taking and fast drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
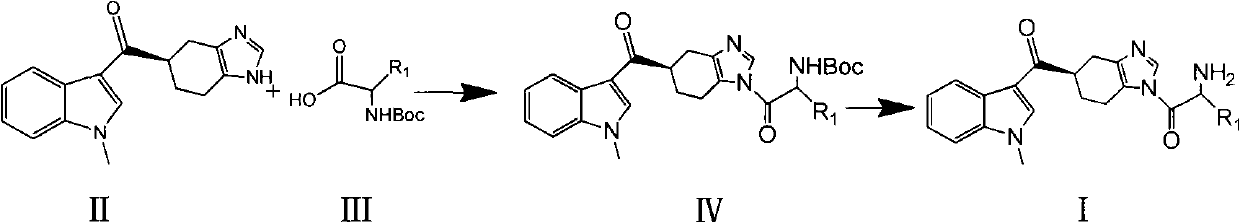
[0050] Example 1 5(R)-[(1-methylindol-3-yl)carbonyl]-1-(tert-butoxyformyl)glycyl-4,5,6,7-tetrahydrobenzimidazole (IV- 1) Preparation
[0051] Take 1g of 5(R)-[(1-methylindol-3-yl)carbonyl]-4,5,6,7-tetrahydrobenzimidazole (II) and 0.5g of Boc-glycine into 50ml In a round bottom flask, add 10ml tetrahydrofuran (THF), stir for 10 minutes, dissolve completely, add 1.4g of 1-hydroxy-benzo-triazole (HOBt), 1g of N,N'-dicyclohexylcarbonyl Amine (DCC), 1ml of triethylamine, dissolved. Under the condition of stirring at room temperature, a white solid gradually precipitated, reacted for 5 hours, the reaction was basically complete, filtered, and the filtrate was evaporated to dryness to obtain an oily substance, added 30ml of ethyl acetate, added 20ml of water, added a small amount of acid water dropwise, and adjusted the pH value to about 4 -5 or so, stirred at room temperature for 0.5 hours, and filtered. The extraction was repeated 3 times with 30 ml of ethyl acetate. The ethy...
Embodiment 2
[0052] Example 2 Preparation of 5(R)-[(1-methylindol-3-yl)carbonyl]-1-glycyl-4,5,6,7-tetrahydrobenzimidazole (I-1)
[0053] Put 1.2 g of the crude product (IV-1) of Example 1 into a 50 ml round-bottomed flask, add 25 ml of dichloromethane, stir for 5 minutes, after complete dissolution, add 1.09 g of trifluoroacetic acid dropwise, and stir at room temperature for 4 hours. Stop the reaction, add 30ml of water, adjust the pH value to greater than 9 with aqueous sodium hydroxide solution, then add 30ml of ethyl acetate, repeat the extraction 3 times, separate the ethyl acetate layer, and dry it with anhydrous magnesium sulfate overnight. The next day, suction filtration under reduced pressure, the filtrate was concentrated to dryness to obtain 0.62 g of a light yellow solid, which was separated by a column to obtain 0.3 g of a light yellow solid (I-1), detected by TCL, R f 0.3 (developing solvent: ethyl acetate / ethanol = 1:1), yield 32.4%. 1 HNMR (DMSO-d 6 ), δ(ppm): 1.5-2.0(...
Embodiment 3-7
[0055] According to the same method as in Example 1, the difference is that (III) compounds of different structures and 5(R)-[(1-methylindol-3-yl)carbonyl]-4,5,6,7-tetrahydro Benzimidazole (II) was reacted, and the reaction temperature and reaction time were prepared with reference to the following Table 1 to obtain the compound of the following formula (IV). The results are shown in Table 1.
[0056] Table 1
[0057] Example
(III)
(IV)
reaction
temperature
reaction
time
code name
3
Boc-preserved
5(R)-[(1-methylindol-3-yl)carbonyl]-1-
(tert-butoxyformyl)prolyl-4,5,6,
7-tetrahydrobenzimidazole
50℃
8 hours
(IV-2)
4
Boc-filament
5(R)-[(1-methylindol-3-yl)carbonyl]-1-
(tert-butoxyformyl)seryl-4,5,6,
7-Tetrahydrobenzimidazole
room temperature
5 hours
(IV-3)
5
Bo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com