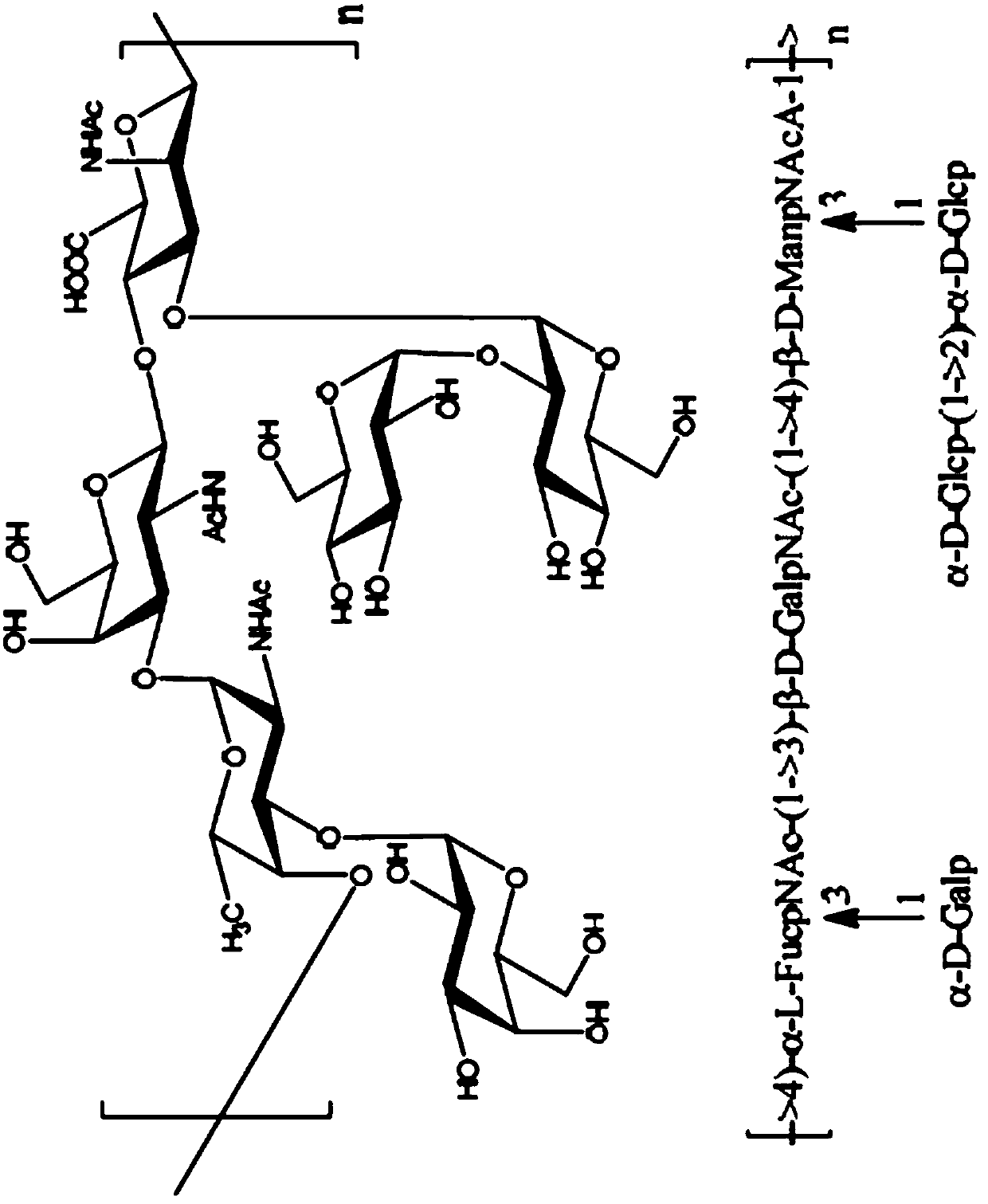
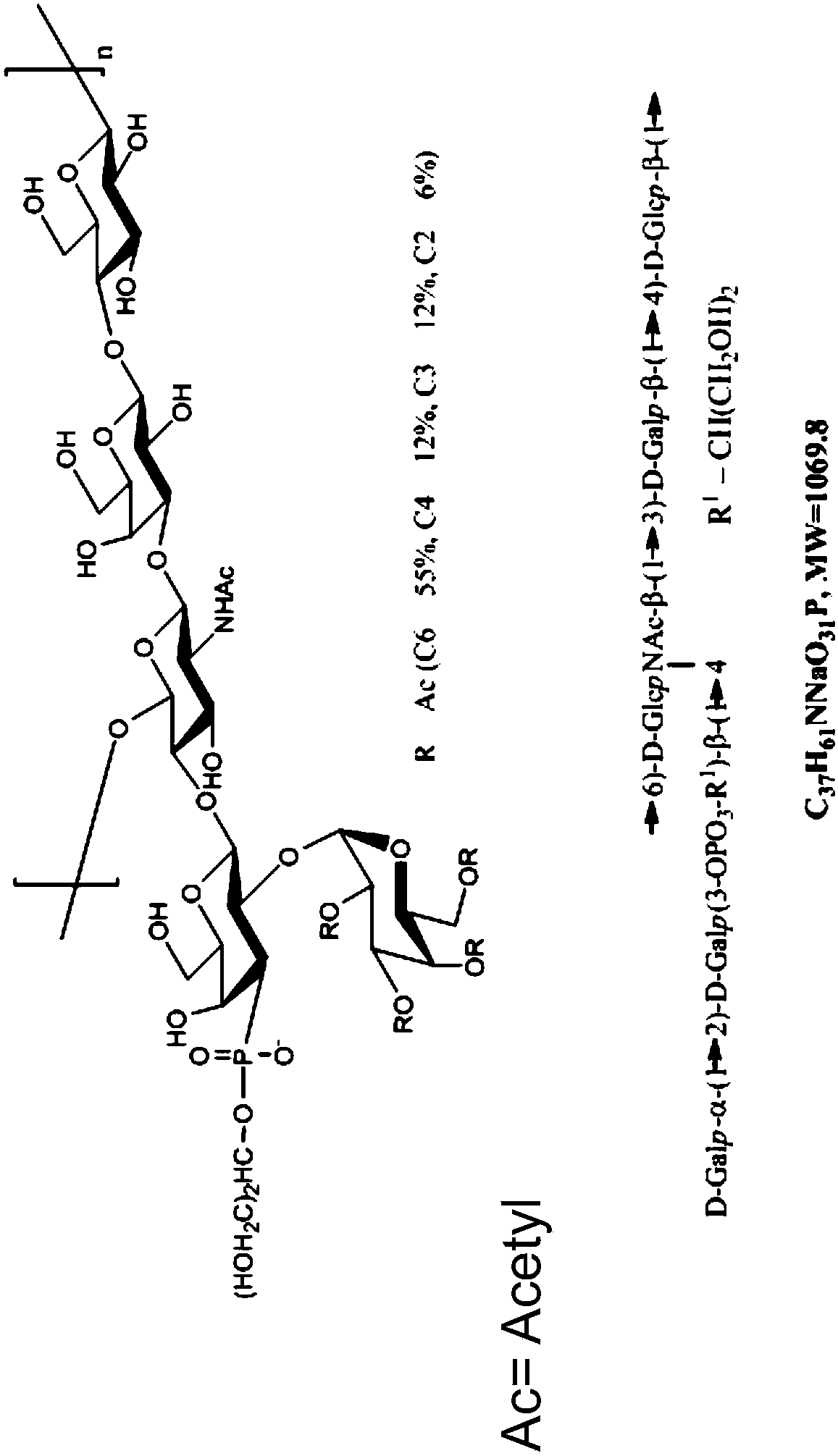
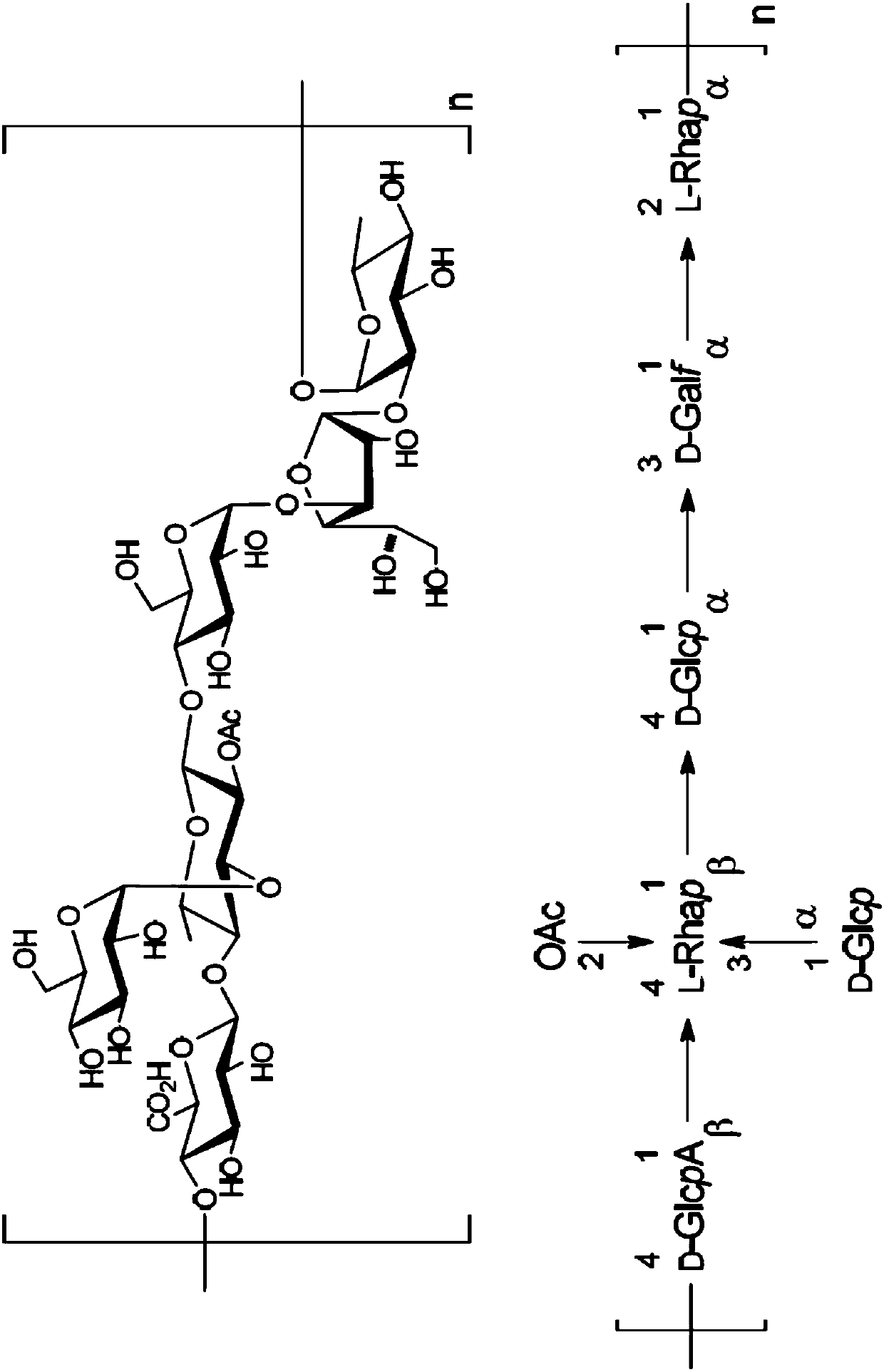
Immunogenic compositions comprising conjugated capsular saccharide antigens, kits comprising the same and uses thereof
An immunogenicity and kit technology, applied in the direction of carrier-bound antigen/hapten components, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as the complexity of multivalent vaccine development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1727] Example 1: General method for preparing eTEC-linked glycoconjugates
[1728] Activation of sugar and thiolation with cystamine dihydrochloride
[1729] Reconstitute the sugar in dry dimethyl sulfoxide (DMSO). The moisture content of the solution is determined by Karl Fischer (KF) analysis and adjusted to achieve a moisture content of 0.1% to 0.4% (usually 0.2%).
[1730] To initiate activation, freshly prepared 1,1'-carbonyl-di-1,2,4-triazole (CDT) or 1,1'-carbonyldiimidazole (CDI) in DMSO at a concentration of 100 mg / mL Solution. Various amounts of CDT / CDI (1-10 molar equivalents) were used to activate the sugar, and the reaction was carried out at 23±2°C for 1 hour. The activation level can be determined by HPLC. Cystine dihydrochloride is freshly prepared at a concentration of 50 mg / mL in anhydrous DMSO. The activated sugar is reacted with 1 molar equivalent (mol.eq.) of cystamine dihydrochloride. Alternatively, the activated sugar is reacted with 1 molar equivalent...
Embodiment 2
[1750] Example 2: Preparation of Pn-33F eTEC conjugate
[1751] Activation method
[1752] Activation of Pn33F polysaccharide
[1753] Pn-33F polysaccharide was mixed with 500 mM 1,2,4-triazole (in WFI) to obtain 10 grams of triazole per gram of polysaccharide. The shell-frozen of the mixture was frozen in a dry ice-ethanol bath and then lyophilized to dryness. The lyophilized 33F polysaccharide was reconstituted in anhydrous dimethyl sulfoxide (DMSO). The moisture content of the lyophilized 33F / DMSO solution was determined by Karl Fischer (KF) analysis. The moisture content was adjusted to a moisture content of 0.2% by adding WFI to the 33F / DMSO solution.
[1754] To initiate activation, 1,1'-carbonyl-di-1,2,4-triazole (CDT) was freshly prepared at 100 mg / mL in DMSO solution. The Pn33F polysaccharide was activated with various amounts of CDT before the thiolation step. CDT activation was performed at 23±2°C for 1 hour. The activation level was determined by HPLC (A220 / A205)....
Embodiment 3
[1778] Example 3: Preparation of additional Pn-33F eTEC conjugate
[1779] Additional Pn-33F eTEC conjugates were produced using the method described in Example 2. The reaction parameters and characterization data of these additional batches of Pn-33F eTEC glycoconjugates are given in Table 3.
[1780] Table 3. Experimental parameters and characterization data of additional Pn33F eTEC conjugates
[1781]
[1782] LOQ = limit of quantification; N / A = not available
[1783] As described above and in Table 3, several Pn-33F conjugates were obtained using the above-mentioned eTEC conjugation. eTEC chemistry makes it possible to prepare conjugates with high yields, low% free sugars and high degree of conjugation (conjugated lysine). In addition, more than 80% of acetyl functionality can be maintained using the eTEC conjugation method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com