16-valent streptococcus pneumoniae conjugate vaccine composition
A Streptococcus pneumoniae vaccine composition technology, applied in the field of 16-valent Streptococcus pneumoniae conjugate vaccine composition, to achieve the effect of increasing broad coverage, broad-spectrum and high-efficiency protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Prepare strain bank
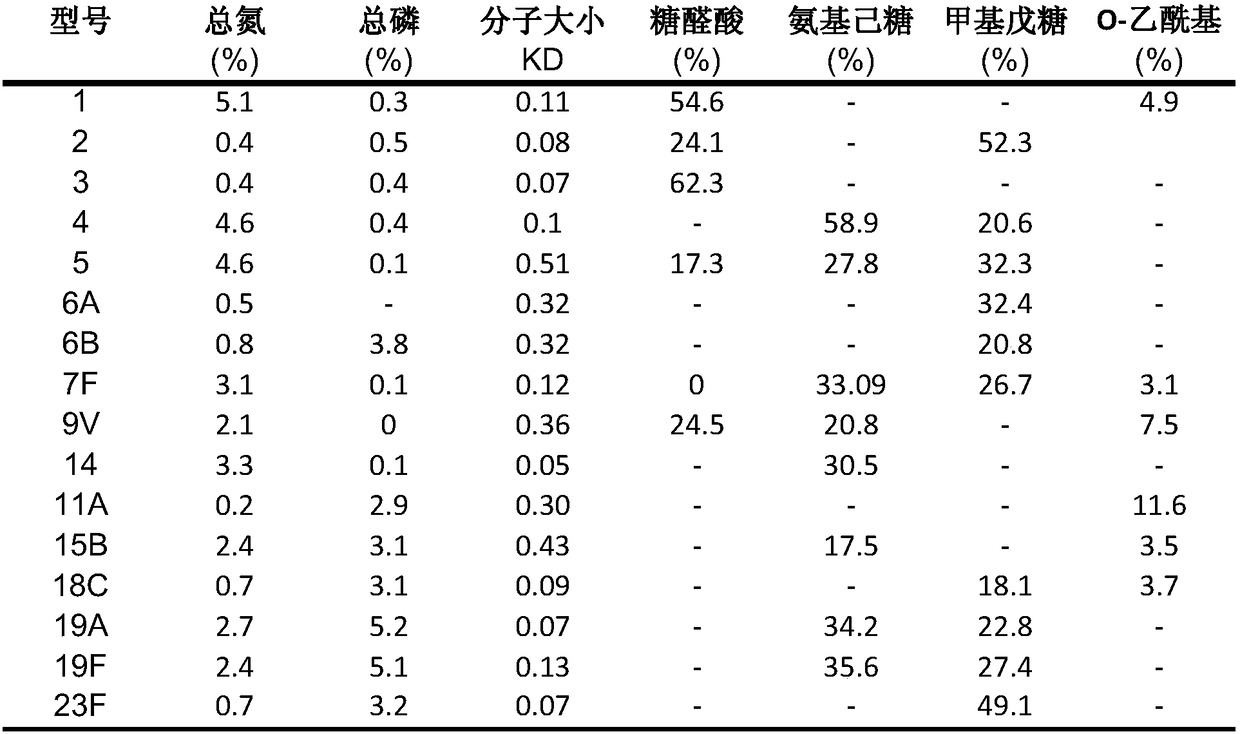
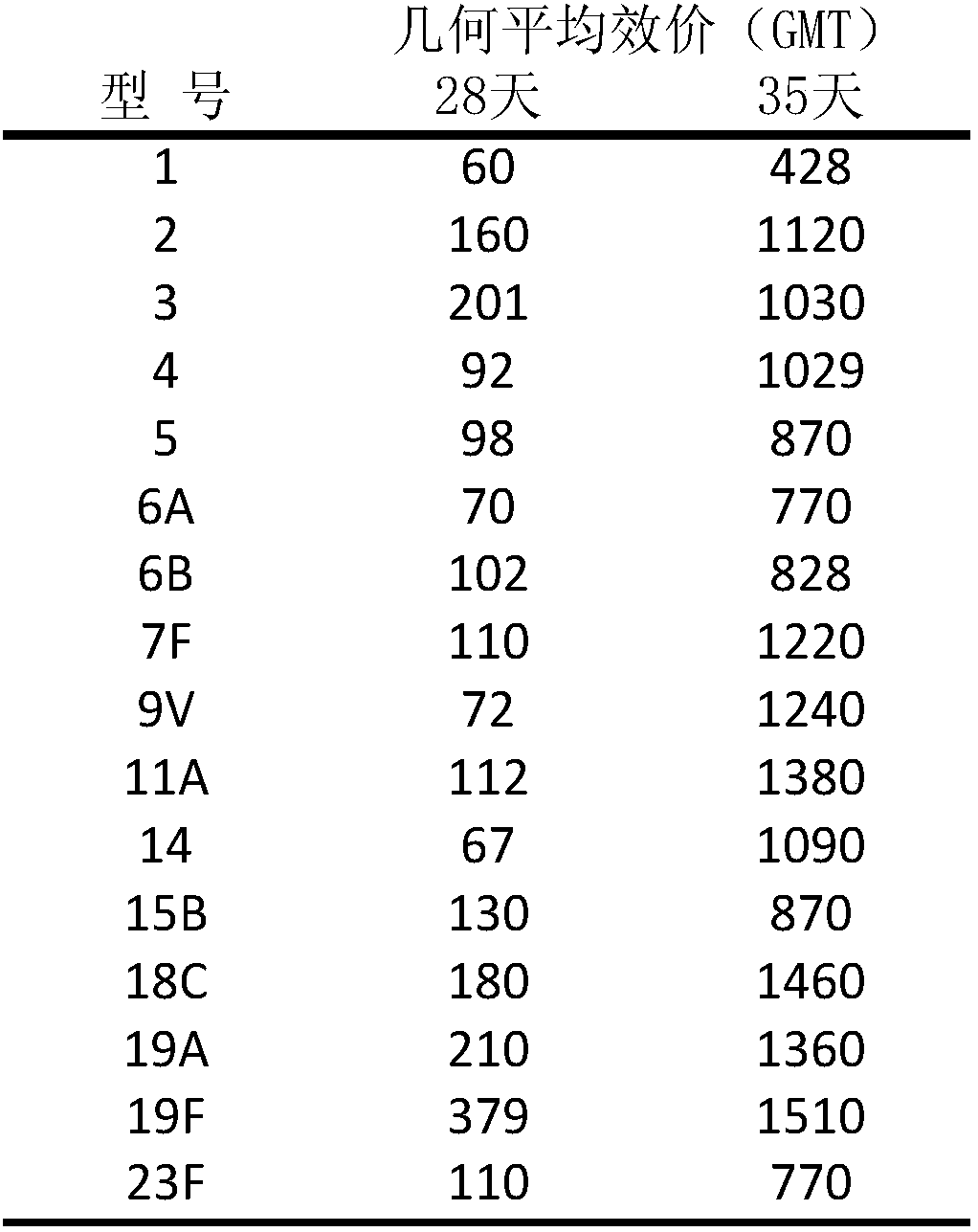
[0022] Different serotypes of Streptococcus pneumoniae were inoculated into fresh sheep blood plates at 36°C, 5% CO 2 Culture and subculture in medium, and then amplify culture in liquid medium. After reaching a certain cell density, prepare the main generation and working generation strain banks, and store them in low-temperature refrigeration. The bacterial strain bank determines the serotype, viability, sterility, stability, etc. of Streptococcus pneumoniae through identification and identification experiments, and is suitable for research and development and large-scale production.
[0023] Fermentation and Harvesting
[0024] Take the working strains and pick the strains by streaking on the solid medium, inoculate them in 5ml of liquid medium, cultivate them at 36°C, transfer them to 500ml of culture medium, and expand them into 10L or 50L fermenters after culture. The fermentation broth is kept at pH 6.5-8.5, the air flow is controlled, and...
Embodiment 2
[0041] Fermentation and Harvesting
[0042] Take the working seeds and pick the strains by streaking on the soybean peptone solid medium, inoculate them in 5ml of liquid comprehensive medium, cultivate them at 36±2°C, transfer them to 500ml of culture medium, and then expand them to 10L of culture medium and 50L fermenter containing soybean medium. Maintain pH 7.4 with sterile sodium carbonate solution, and add appropriate amount of sterile sodium deoxycholate at the end of the logarithmic growth phase to lyse bacterial cells and release bacterial capsular polysaccharides. After lysis, cool the fermenter. The pH of the lysed broth was adjusted to about pH 6.0 with acetic acid. Lysates were clarified by continuous flow centrifugation followed by depth filtration and 0.45 μm microfiltration.
[0043] Polysaccharide purification
[0044] The purification of pneumococcal polysaccharide consists of several steps of concentration / ultrafiltration operation, column chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com