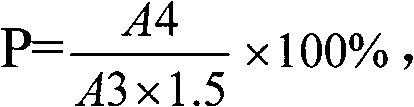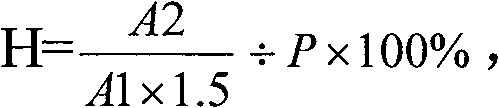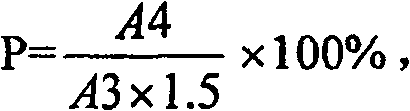Method for determining dissociation amylase content of pneumonia streptococcus capsular polysaccharide combo
A technology of Streptococcus pneumoniae and capsular polysaccharide, applied in the biological field, can solve the problem of inability to accurately measure the content of free polysaccharides in the capsular polysaccharide conjugate of Streptococcus pneumoniae
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take Streptococcus pneumoniae type 1 capsular polysaccharide conjugate as sample 1; take the derivative solution corresponding to the test Streptococcus pneumoniae type 1 capsular polysaccharide conjugate as sample 3.
[0021] Take another 4.5 ml of Streptococcus pneumoniae type 1 capsular polysaccharide conjugate and place it in a 50 ml centrifuge tube; take 4.5 ml of the derivative solution corresponding to the Streptococcus pneumoniae type 1 capsular polysaccharide conjugate and place it in another 50 ml centrifuge tube. Add 1.125ml of 5mol / L sodium chloride solution to each centrifuge tube, shake and mix, then add 22.5ml of absolute ethanol, shake and mix, set at -20℃ for 70 hours; centrifuge at 5℃, 4500rpm for 60 minutes, discard the supernatant liquid.
[0022] Add 0.75ml water for injection (that is, 0% ethanol) to each centrifuge tube, shake and mix, and let stand at room temperature for 1 hour; then add 2.25ml of water for injection, shake and mix, and stand at room...
Embodiment 2
[0030] Take Streptococcus pneumoniae type 2 capsular polysaccharide conjugate as sample 1, and take the derivative solution corresponding to the test Streptococcus pneumoniae type 2 capsular polysaccharide conjugate as sample 3.
[0031] Take another 4.5ml of Streptococcus pneumoniae type 2 capsular polysaccharide conjugate and place it in a 50ml centrifuge tube; take 4.5ml of the derivative solution corresponding to the Streptococcus pneumoniae type 2 capsular polysaccharide conjugate and place it in another 50ml centrifuge tube. Add 1.125ml of 5mol / L sodium chloride solution to each centrifuge tube, shake and mix, then add 22.5ml of absolute ethanol, shake and mix, set at -20°C for 90 hours; centrifuge at 5°C, 6500rpm for 90 minutes, discard the supernatant liquid.
[0032] Add 0.75ml 60% ethanol solution to each centrifuge tube, shake and mix, and let stand at room temperature for 1 hour; then add 2.25ml 60% ethanol solution, shake and mix, and stand at room temperature for 2 ho...
Embodiment 3
[0040] Take Streptococcus pneumoniae type 3 capsular polysaccharide conjugate as sample 1, and take the derivative solution corresponding to the test Streptococcus pneumoniae type 3 capsular polysaccharide conjugate as sample 3.
[0041] Take another 4.5ml of Streptococcus pneumoniae type 3 capsular polysaccharide conjugate and place it in a 50ml centrifuge tube; take 4.5ml of the derivative solution corresponding to Streptococcus pneumoniae type 3 capsular polysaccharide conjugate and place it in another 50ml centrifuge tube. Add 1.125ml of 5mol / L sodium chloride solution to each centrifuge tube, shake and mix, then add 22.5ml of absolute ethanol, shake and mix, set at -20℃ for 90 hours; centrifuge at 5℃, 5500rpm for 70 minutes, discard the supernatant liquid.
[0042] Add 0.75ml 40% ethanol solution to each centrifuge tube, shake and mix, and stand at room temperature for 1 hour; then add 2.25ml 40% ethanol solution, shake and mix, and stand at room temperature for 2 hours; centr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com