A kind of pneumococcal polysaccharide protein conjugated vaccine and preparation method thereof
A pneumococcal polysaccharide and pneumococcal technology, applied in the field of immunology, can solve the problems of transient immune response, reduced immunogenicity, and low immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
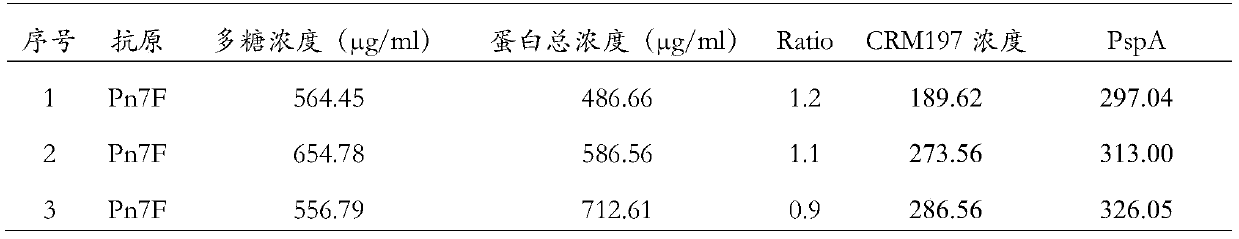
[0049] Example 1 Preparation of a 7F-type capsular polysaccharide and two different carrier protein conjugates
[0050] Dissolve 5g of the lung chain 7F type polysaccharide in 1L of sodium acetate buffer (50mMpH4.5), at room temperature, stir for 20 minutes with a magnetic stirrer with a magnetic sub, in order to fully dissolve the polysaccharide, add 0.2gNaIO 4 , and react overnight at room temperature, so that the adjacent dihydroxyl groups on the polysaccharide are oxidized into aldehyde groups. Perform 20 equal-volume ultrafiltration changes with purified water, and remove the remaining NaIO in the reaction 4 And the small molecules generated in the reaction process were filtered off to obtain an aqueous solution in which the 7F activated polysaccharide with an activation degree of 6.2 was introduced; and the activated polysaccharide could be concentrated and freeze-dried. The molecular size of the activated polysaccharide was determined using a gel filtration chromatogra...
Embodiment 2
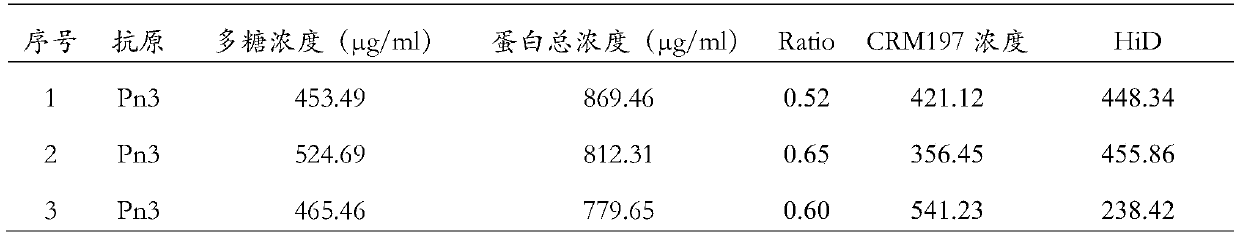
[0055] Example 2 Preparation of a type 3 capsular polysaccharide and two different carrier protein conjugates
[0056] Dissolve 2g of lung chain type 3 polysaccharide in 1L of sodium phosphate buffer (50mMpH7.2), stir for 20 minutes with a magnetic stirrer with a magnet at room temperature, to fully dissolve the polysaccharide, add 0.2gNaIO 4 , and react overnight at room temperature, so that the adjacent dihydroxyl groups on the polysaccharide are oxidized into aldehyde groups. Perform 20 equal-volume ultrafiltration changes with purified water, and remove the remaining NaIO in the reaction 4 and the small molecular substances generated during the reaction were filtered off to obtain an aqueous solution in which the type 3 activated polysaccharide with an activation degree of 9.1 was introduced; and the activated polysaccharide was concentrated and freeze-dried. The molecular size of the activated polysaccharide was determined using a gel filtration chromatography column usi...
Embodiment 3
[0062] Example 3 Preparation of a 13-valent pneumococcal polysaccharide-protein conjugate vaccine
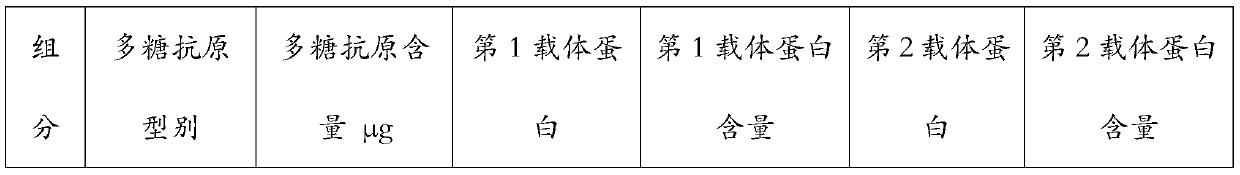
[0063]Single-carrier conjugates such as 4, 6B, 9V, 14, 18C, 19F, 23F were prepared according to known methods in the background art, wherein the carrier proteins were all CRM197. According to the method described in Example 1 or 2, prepare 1,3,5,6A, 7F,19A type polysaccharide double carrier conjugates, wherein 1,5,7F type polysaccharide adopts PspA as the second carrier, 3,6A,19A HiD was used as the second vector. The various conjugated products were formulated according to Table 3 below. After mixing these monovalent components, aluminum phosphate adjuvant was added at a final concentration of 0.5 mg / L.
[0064] Table 3 The carrier protein type of various antigens and the final content in the preparation
[0065]
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com