Method for monitoring tetanus toxoid or diphtheria toxoid
A technology for tetanus toxoid and diphtheria toxoid, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems that there is no method for evaluating or monitoring formaldehyde detoxification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
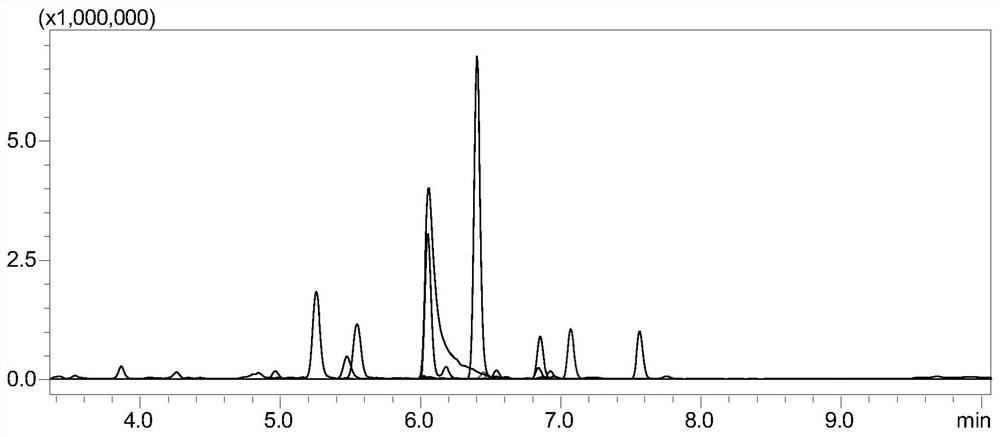
[0087] The monitoring of embodiment 1 tetanus toxoid
[0088] 1. Experimental instruments and equipment: high-pressure binary pump, degasser, autosampler, column thermostat and triple quadrupole mass spectrometer.
[0089] 2. Experimental reagents: standard tetanus toxoid, dithiothreitol (DTT), iodoacetamide (IAA), ammonium bicarbonate, RapiGest TM , trypsin, tetanus toxoid samples.
[0090] 3. Detection conditions:
[0091] Chromatographic conditions:
[0092] Chromatographic column: stationary phase 1 (biocompatible C18 chromatographic column);
[0093] Mobile phase: A: acetic acid aqueous solution (the volume ratio of acetic acid to water is 1:1000); B: acetic acid-acetonitrile mixture (the volume ratio of acetic acid to acetonitrile is 1:1000);
[0094] Gradient: 0~8min, 5%B~40%B; 8~8.1min, 40%B~100%B; 8.1~10min, 100%B; 10~10.1min, 100%B~5%B; 10.1~ 15min, 5% B; column temperature: 35°C; flow rate: 0.2-0.5mL / min; injection volume: 10μL.
[0095] Mass Spectrometry Cond...
Embodiment 2
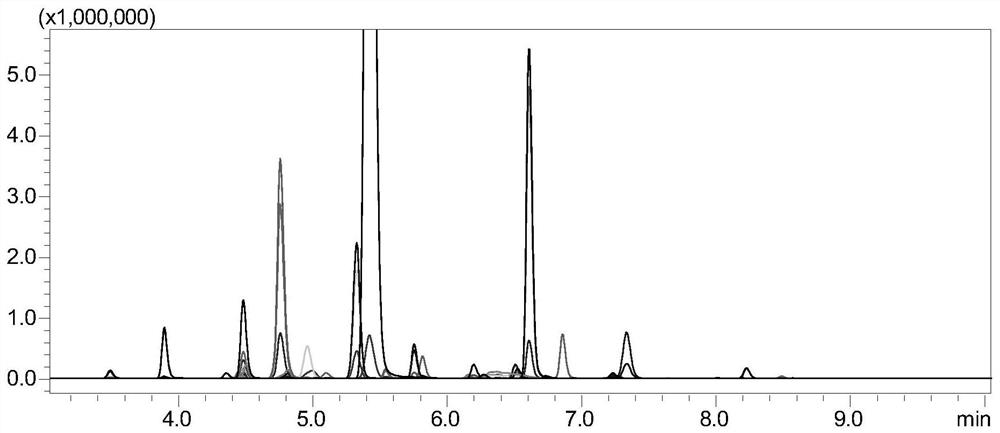
[0121] The monitoring of embodiment 2 tetanus toxoid
[0122] 1. Experimental instruments and equipment: high-pressure binary pump, degasser, autosampler, column thermostat and triple quadrupole mass spectrometer.
[0123] 2. Experimental reagents: standard tetanus toxoid, dithiothreitol (DTT), iodoacetamide (IAA), ammonium bicarbonate, RapiGest TM , chymotrypsin, tetanus toxoid samples.
[0124] 3. Detection conditions:
[0125] Chromatographic conditions:
[0126] Chromatographic column: stationary phase 1 (biocompatible C18 chromatographic column);
[0127] Mobile phase: A: acetic acid aqueous solution (the volume ratio of acetic acid to water is 1:1000); B: acetic acid-acetonitrile mixture (the volume ratio of acetic acid to acetonitrile is 1:1000);
[0128] Gradient: 0~8min, 5%B~40%B; 8~8.1min, 40%B~100%B; 8.1~10min, 100%B; 10~10.1min, 100%B~5%B; 10.1~ 15min, 5% B; column temperature: 35°C; flow rate: 0.2-0.5mL / min; injection volume: 10μL.
[0129] Mass Spectrometry...
Embodiment 3
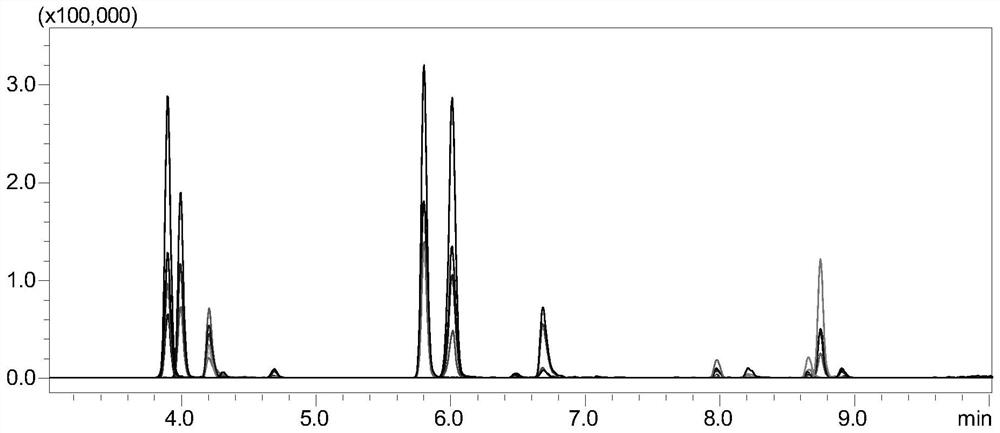
[0156] Example 3 Monitoring of diphtheria toxoid
[0157] 1. Experimental instruments and equipment: high-pressure binary pump, degasser, autosampler, column thermostat and triple quadrupole mass spectrometer.
[0158] 2. Experimental reagents: diphtheria toxoid standard, dithiothreitol (DTT), iodoacetamide (IAA), ammonium bicarbonate, RapiGest TM , trypsin, diphtheria toxoid samples.
[0159] 3. Detection conditions:
[0160] Chromatographic conditions:
[0161] Chromatographic column: stationary phase 1 (biocompatible C18 chromatographic column);
[0162] Mobile phase: A: acetic acid aqueous solution (the volume ratio of acetic acid to water is 1:1000); B: acetic acid-acetonitrile mixture (the volume ratio of acetic acid to acetonitrile is 1:1000);
[0163] Gradient: 0~8min, 5%B~40%B; 8~8.1min, 40%B~100%B; 8.1~10min, 100%B; 10~10.1min, 100%B~5%B; 10.1~ 15min, 5% B; column temperature: 35°C; flow rate: 0.2-0.5mL / min; injection volume: 10μL.
[0164] Mass Spectrometry Co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com