Multivalent DTP-POLID vaccines
An immune composition and technology for polio, applied in the field of multivalent vaccine administered in pediatrics, can solve the problem of immunogenicity reduction and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
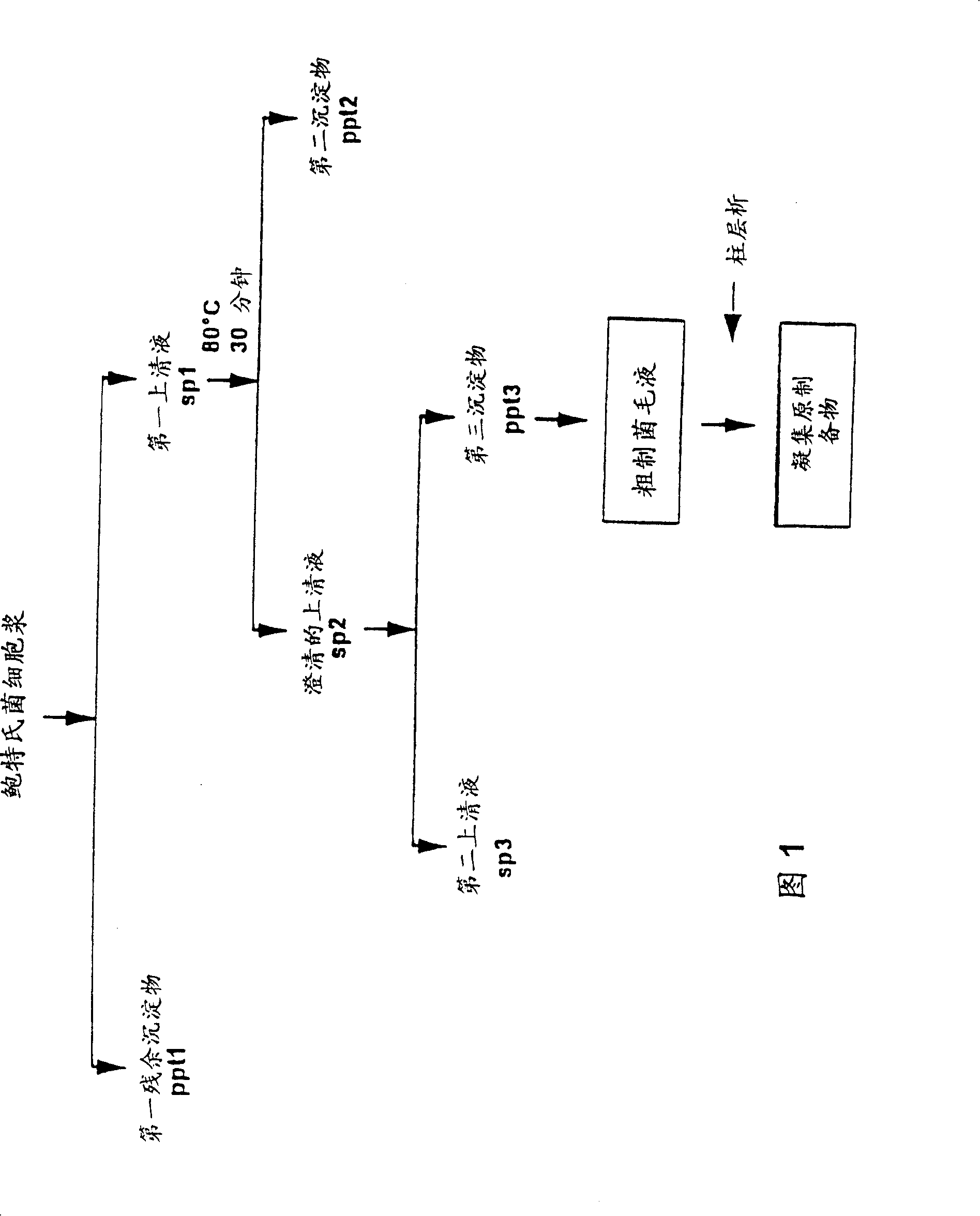
[0072] Agglutinogen preparation
[0073] Referring to Fig. 1, this is a flowchart illustrating a method for preparing an agglutinogen from a Bordetia strain. As shown in Figure 1, a Bordetella cell homogenate containing an agglutinogen, such as a Bordetella pertussis cell homogenate, is extracted with, for example, a urea-containing buffer, such as selection with 10 mM potassium phosphate, 150 mM NaCl and 4M urea. The agglutinogen was selectively extracted from the cell homogenate to obtain the first supernatant (sp1) containing the agglutinogen and the first residual precipitate (ppt1). The first supernatant (sp1) can be separated from the first residual precipitate (ppt1) by eg centrifugation. This residual precipitate (ppt1) was discarded. The clarified supernatant (sp1) can then be concentrated and diafiltered eg 10 mM potassium phosphate / 150 mM NaCl / 0.1% Triton X-100 using eg a 100-300 kDa NMWL filter.
[0074] The first supernatant is then incubated at a certain tempe...
Embodiment 1
[0223] This example will describe the cultivation of Bordetella pertussis.
[0224] Main strain:
[0225] The culture of the main strain of Bordetella pertussis is stored at 2°C-8°C in the form of batch freeze-dried strains.
[0226] Working strains:
[0227] This freeze-dried culture was revived in Hornibrook medium and used to plate on Bordet-Gengou agar (BGA) plates. Hornibrook medium contains the following components:
[0228] Composition 1 Litre
[0229] Casein hydrolyzate (activated carbon treatment) 10.0g
[0230] Niacin 0.001g
[0231] CaCl 2 0.002g
[0232] NaCl 5.0g
[0233] MgCl 2 ·6H 2 O 0.025g
[0234] KCl0.200g
[0235] K 2 HPO 4 0.250g
[0236] Starch 1.0g
[0237] distilled water to 1.0 liter
[0238] Adjust the pH to 6.9 ± 0.1 with 1% sodium carbonate. This medium was dispensed into test tubes and sterilized: steam heated in an autoclave for 20 minutes, then autoclaved at 121°C-124°C for 20 minu...
Embodiment 2
[0267] This example describes the purification of antigens from Bordetella pertussis cell cultures.
[0268] Prepare media and cell concentrates:
[0269] In two production fermenters, the bacterial suspension was incubated at 34°C-37°C for 35-50 hours. Take samples from the fermenter and check that there are no bacteria in the culture medium. This suspension was added to a continuous flow disc-pack centrifuge and centrifuged (12000 xg) to separate the cells from the culture medium. Cells are collected and ready for extraction of pili components. The supernatant was passed through a ≤0.22 μm filter membrane, and the filtrate was concentrated by ultrafiltration with a 10-30 kDa nominal molecular weight limit (NMWL) membrane. Store this concentrate, waiting for the separation and purification of pertussis toxin (PT), filamentous hemagglutinin and 69kDa (Pertactin) and other components.
[0270] Separate the components in the culture medium:
[0271] The components (69 kDa, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com