Porcine pseudorabies virus virulent strain, and gene deletion vaccine strain thereof and applications thereof
A gene deletion vaccine, porcine pseudorabies technology, applied in the direction of antiviral agents, viruses/phages, microorganism-based methods, etc., can solve the problem that the new virus cannot be completely resisted, the newly isolated virus cannot be effectively neutralized, and the immunized pig cannot effectively resist Virus and other problems, to achieve the effect of facilitating differential diagnosis, high protection efficiency and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
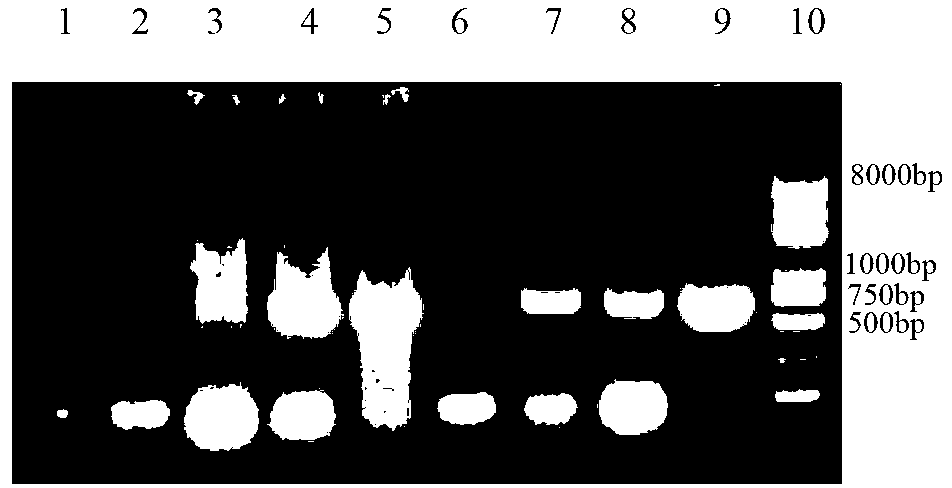
[0033] Example 1 Isolation and identification of porcine pseudorabies virus virulent strain (PRVHeN1 strain)
[0034] The brain tissue of diseased pigs was collected from a pig farm suspected of PRV infection in Henan Province, my country, and the tissue genomic DNA was extracted, and PCR identification was carried out using PRVgE-specific primers ( figure 1 ), the positive brain tissue homogenate was centrifuged, filtered and the filtrate was frozen for later use. After Vero cells were cultured at 37°C for 48 hours to form a monolayer, each bottle was inoculated with 1ml of the supernatant of the filtered disease material suspension, and after adsorption for 1 hour, the DMEM culture medium containing 2% fetal bovine serum was changed to continue culturing at 37°C. 3 to 4 days, whether there is cytopathy ( figure 2 ). When 60-70% of the cells have CPE changes, the virus is collected, and the virus strains with lesions are passed on for 2 to 3 generations, and then PCR ampli...
Embodiment 2
[0035] Example 2 There are antigenic differences between the PRV HeN1 strain and the vaccine strain Barthak61
[0036] 1) Microneutralization test and sheep immune challenge test were used to confirm the antigenic difference between the newly isolated strain (PRVHeN1 strain) and the vaccine strain Barthak61. Neutralization test operation method: 12 40-day-old SPF pigs, 5 of them intramuscularly injected 1mL containing 10 7.0 TCID 50 The inactivated PRVHeN1 strain, intramuscularly inject 1mL containing 10 7.0 TCID 50 The other 2 heads were injected with DMEM culture medium as a control, and blood was collected every week before and after inoculation to separate serum, and they were killed 5 weeks after inoculation. The neutralizing potency of the serum from pigs inoculated with Barthak61 and HeN1 against two strains of viruses (Barthak61 and HeN1) was determined by the fixed virus dilution serum method. The virus volume is 100TCID 50 / well, the serum was collected at diffe...
Embodiment 3
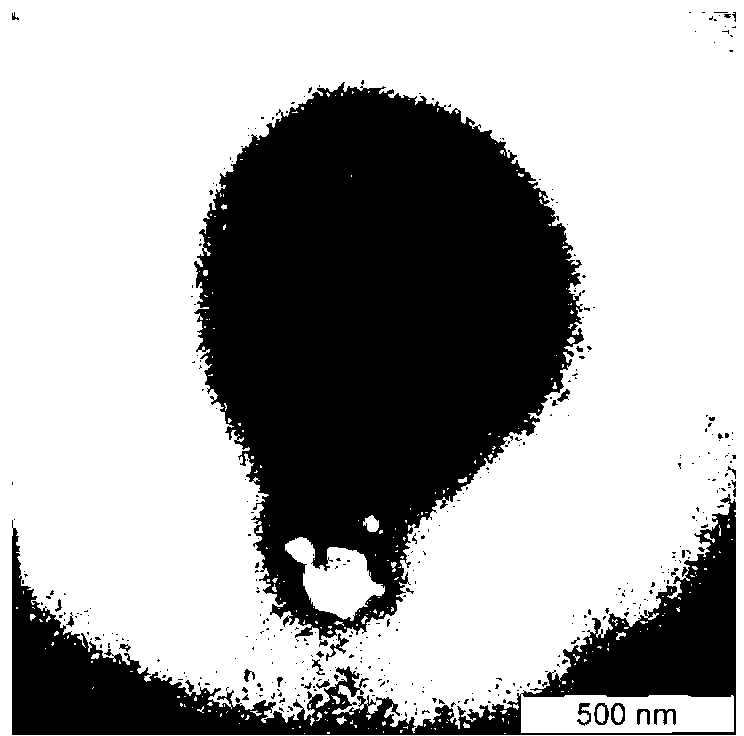
[0040] Example 3 Gene Deletion Vaccine Strain rPRV-gE - -EGFP + build
[0041] The transfer vector plasmid pUCUsAB-EGFP was constructed by gene cloning technology using part of the gI and Us2 genes of the PRVHeN1 strain as homology arms, and the green fluorescent protein gene (EGFP) as a screening marker (recombination sites such as Figure 5 As shown, the enzyme digestion of the transfer vector was identified as Image 6 ), the PRVHeN1 genome and the transfer vector were co-transfected into Vero cells, according to the instructions of the calcium phosphate transfection kit, the Vero cells were pre-cultured in a 60mm dish to form a monolayer, and the fresh culture medium was replaced 3 hours before transfection, Mix 4 μg viral genomic DNA and 10 μg transfer vector pUCUsAB-EGFP, then add 2MCaCl 2 , so that the final concentration of 0.24M, mixed evenly, this DNA-CaCl 2 The mixture was then added to a new tube containing an equal volume of 2XHBS, mixed well, and allowed to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com