Diphtheria, tetanus and acellular pertussis/Haemophilus influenzae type b - group A and C meningococcus combined vaccine
A cell-free DPT and Haemophilus influenzae technology, applied in antibacterial drugs, bacterial antigen components, antibody medical components, etc., can solve the problems of complex vaccine components, interference of detection methods, interference of free polysaccharide detection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
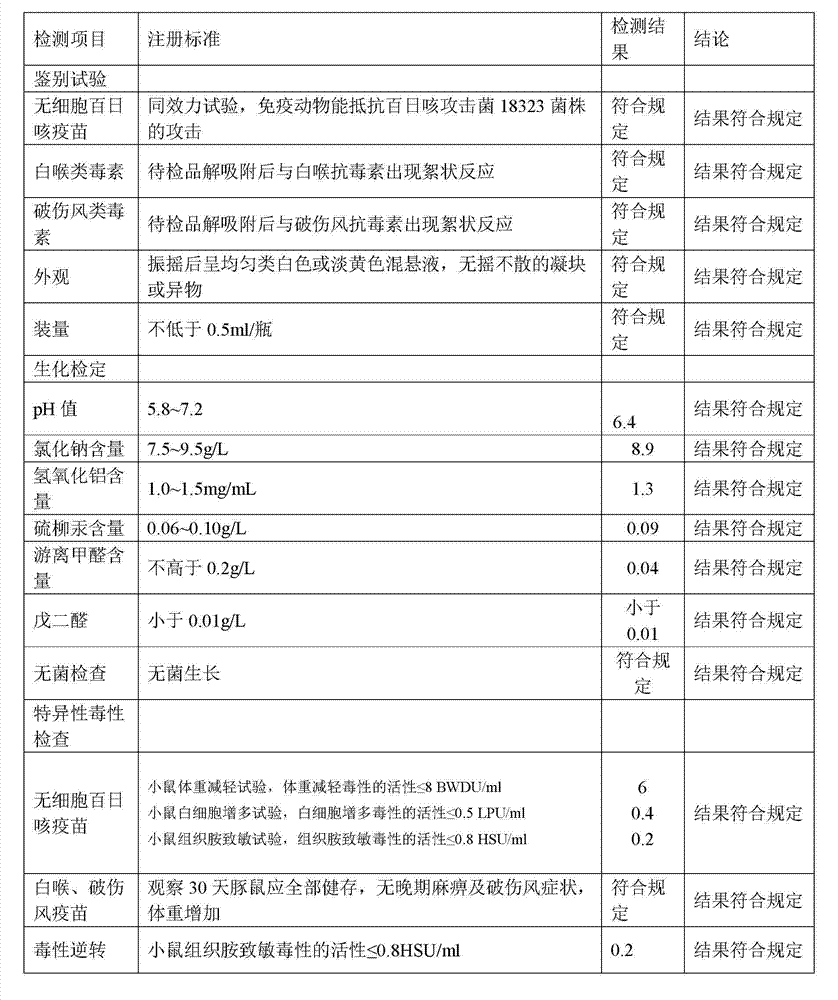
[0057] 1. Preparation of Adsorbed Acellular DPT Vaccine:
[0058] (1) Prepare each monovalent vaccine stock solution:
[0059] (1) Pertussis vaccine stock solution is manufactured according to the 2010 edition of "Chinese Pharmacopoeia" "Adsorbed DPT combined vaccine" in Appendix 1 "Pertussis vaccine stock solution manufacturing and verification requirements" working seed batch through serological test, skin necrosis test, toxicity test and Freeze-dried after titer determination. After opening the batch of working seed strains, inoculate them on the culture medium, such as the improved bag-ginger medium or activated carbon semi-comprehensive medium, and cultivate them at 35~37°C for no more than 72 hours. After the pure bacteria inspection, collect the bacteria and suspend them. In PBS of pH7.0~7.4. Use formaldehyde solution with a final concentration of less than 0.1% to sterilize, and after the sterilization test, the pertussis vaccine stock solution is obtained.
[0060]...
Embodiment 2
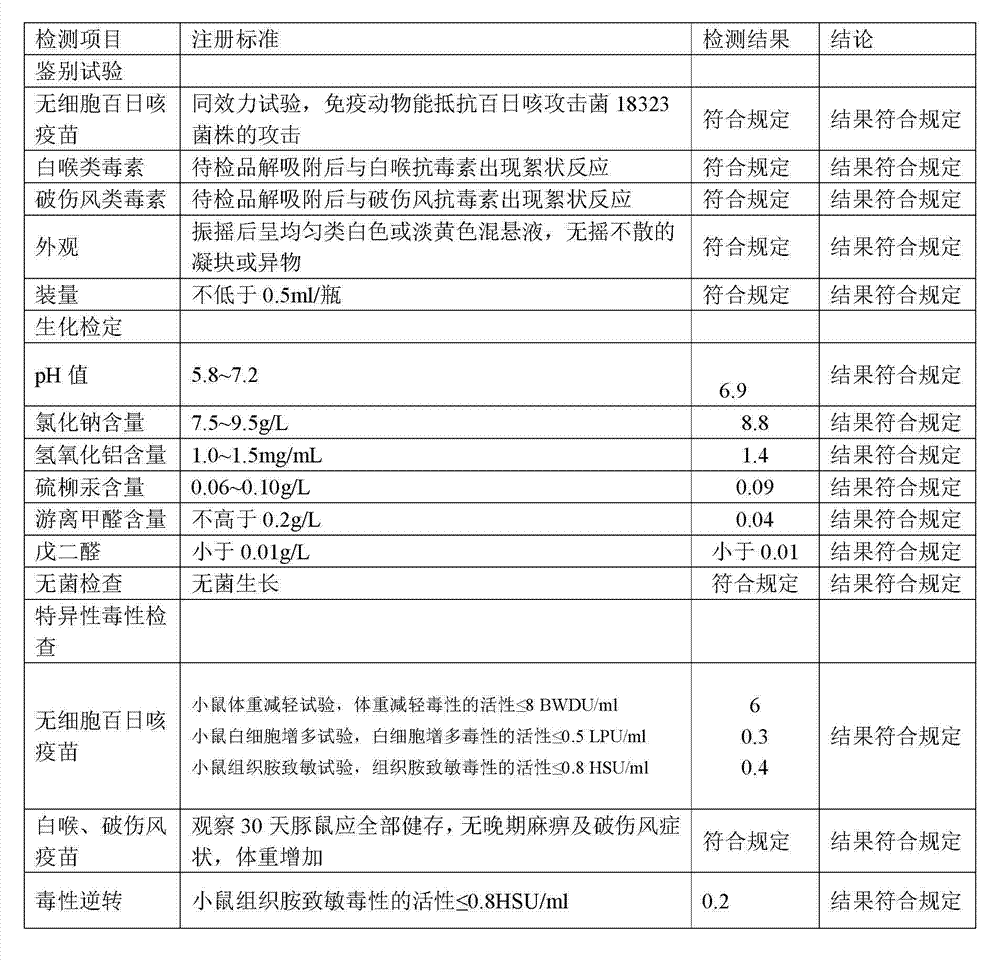
[0104] 1. Preparation of Adsorbed Acellular DPT Vaccine:
[0105] (1) Prepare each monovalent vaccine stock solution:
[0106] (1) Pertussis vaccine stock solution is manufactured according to the 2010 edition of "Chinese Pharmacopoeia" "Adsorbed DPT combined vaccine" in Appendix 1 "Pertussis vaccine stock solution manufacturing and verification requirements" working seed batch through serological test, skin necrosis test, toxicity test and Freeze-dried after titer determination. After opening the batch of working seed strains, inoculate them on the culture medium, such as the improved bag-ginger medium or activated carbon semi-comprehensive medium, and cultivate them at 35~37°C for no more than 72 hours. After the pure bacteria inspection, collect the bacteria and suspend them. In PBS of pH7.0~7.4. Use formaldehyde solution with a final concentration of less than 0.1% to sterilize, and after the sterilization test, the pertussis vaccine stock solution is obtained.
[0107]...
Embodiment 3
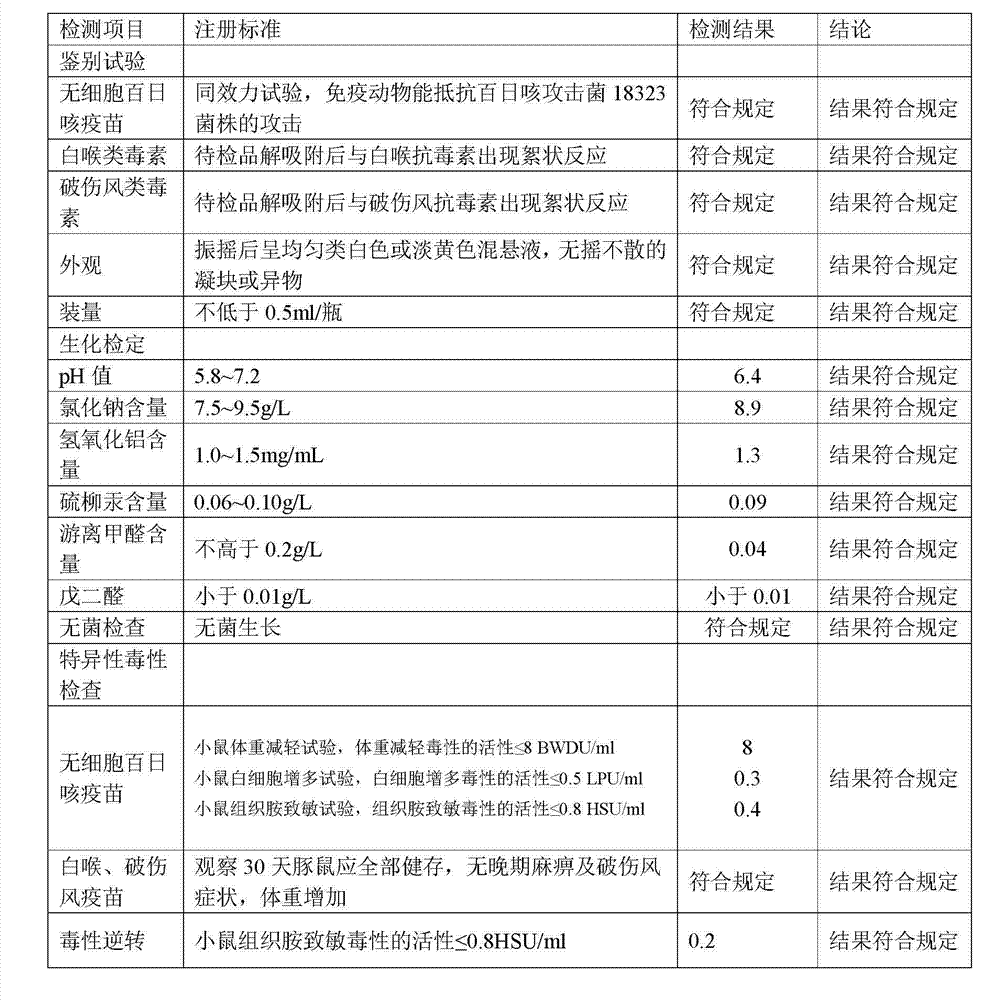
[0152] 1. Preparation of Adsorbed Acellular DPT Vaccine:
[0153] (1) Prepare each monovalent vaccine stock solution:
[0154] (1) Pertussis vaccine stock solution is manufactured according to the 2010 edition of "Chinese Pharmacopoeia" "Adsorbed DPT combined vaccine" in Appendix 1 "Pertussis vaccine stock solution manufacturing and verification requirements" working seed batch through serological test, skin necrosis test, toxicity test and Freeze-dried after titer determination. After opening the batch of working seed strains, inoculate them on the culture medium, such as the improved bag-ginger medium or activated carbon semi-comprehensive medium, and cultivate them at 35~37°C for no more than 72 hours. After the pure bacteria inspection, collect the bacteria and suspend them. In PBS of pH7.0~7.4. Use formaldehyde solution with a final concentration of less than 0.1% to sterilize, and after the sterilization test, the pertussis vaccine stock solution is obtained.
[0155]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com