HM-3 polypeptide freeze-dried powder preparation and preparation method thereof
A technology of HM-3 and freeze-dried powder, which is applied in the direction of freeze-drying transportation, powder transportation, pharmaceutical formulations, etc. It can solve the problems of increased impurities, high moisture in products, poor stability, etc. protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Taking 1000 bottles as an example, the formula of HM-3 polypeptide freeze-dried powder preparation is as follows, in parts by weight, 5-100 parts of HM-3 polypeptide, 20-90 parts of excipients, 1-10 parts of pH regulator, of which HM-3 polypeptide The pH of the freeze-dried powder preparation is 5-7.
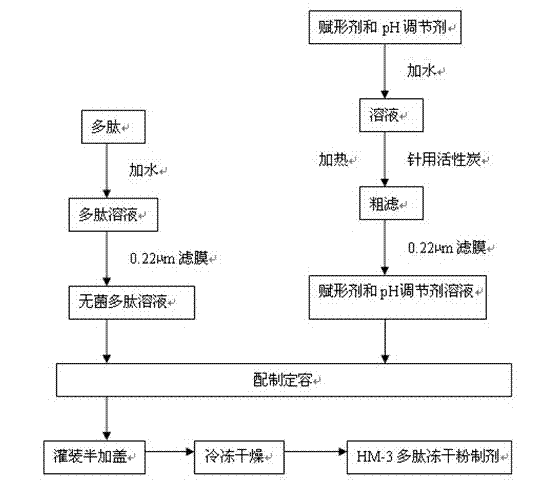
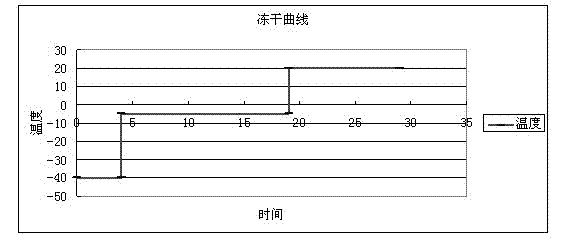
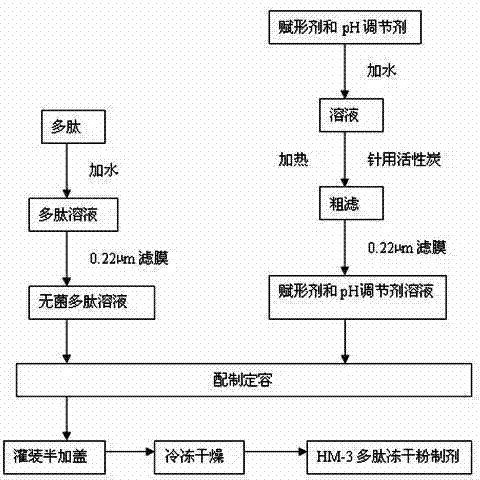
[0023] The production process for preparing the HM-3 polypeptide freeze-dried powder preparation is as follows: figure 1 shown. Dosing: Weigh the HM-3 raw material of the above formula, add 100~4000 parts of water for injection to make a solution, filter it with a 0.22μm membrane aseptically before use. Weigh the excipients and pH regulator of the above formula and add 100~4000 parts of water for injection to make a solution, add 0.01%~5% activated carbon for needles, heat and filter, decarbonize, and filter with 0.22μm membrane to make pyrogen-free aseptic excipients aqueous solution. After cooling, mix the polypeptide solution and the auxiliary material solution, t...
Embodiment 2
[0025] Taking 1000 bottles as an example, the formulation of the HM-3 polypeptide freeze-dried powder preparation is, in parts by weight, 30 parts of HM-3 peptide, 30 parts of dextran, and 10 parts of sodium acetate. The pH of the HM-3 polypeptide freeze-dried powder preparation is 5-7;
[0026] The production process for preparing the HM-3 polypeptide freeze-dried powder preparation is as follows: figure 1shown. Taking 1000 bottles as an example, first prepare the solution, weigh the raw material of HM-3 according to the above parts by weight, add 100 parts of water for injection to make a solution, and filter it with a 0.22 μm membrane for sterile use. Weigh the dextran in the above parts by weight, add 400 parts of water for injection to sodium acetate to form a solution, add 1% activated carbon for needles, heat and filter, decarbonize, and filter with a 0.22 μm membrane to make a pyrogen-free sterile auxiliary material aqueous solution. Mix the polypeptide solution and...
Embodiment 3
[0028] Taking 1000 bottles as an example, the formula of the HM-3 polypeptide freeze-dried powder preparation is as follows, in parts by weight: 30 parts of HM-3 polypeptide, 65 parts of mannitol, 5 parts of sodium hydroxide, and the HM-3 polypeptide freeze-dried powder preparation pH 5-7
[0029] The production process for preparing the HM-3 polypeptide freeze-dried powder preparation is as follows: figure 1 shown. Taking 1,000 bottles as an example, first prepare the solution, weigh the HM-3 raw material in the above parts by weight, add 2,000 parts of water for injection to make a solution, and filter it with a 0.22 μm membrane for sterile filtration. Weigh the mannitol in the above parts by weight, add 3000 parts of water for injection into sodium hydroxide to form a solution, add 0.5% activated carbon for needles, heat and filter, decarbonize, and filter with a 0.22 μm membrane to make a pyrogen-free sterile auxiliary material aqueous solution. Mix the polypeptide solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com