Polyvalent pneumococcus and B-type haemophilus influenzae combined vaccine
A technology for Haemophilus influenzae and pneumococcus, which can be used in multivalent vaccines, vaccines, antibacterial drugs, etc., can solve the problems of high sensitivity of infectious pathogens, inability to play a role, and difficult to promote, and achieves elimination of allergens and easy solubility. Good and easy vaccine transport effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
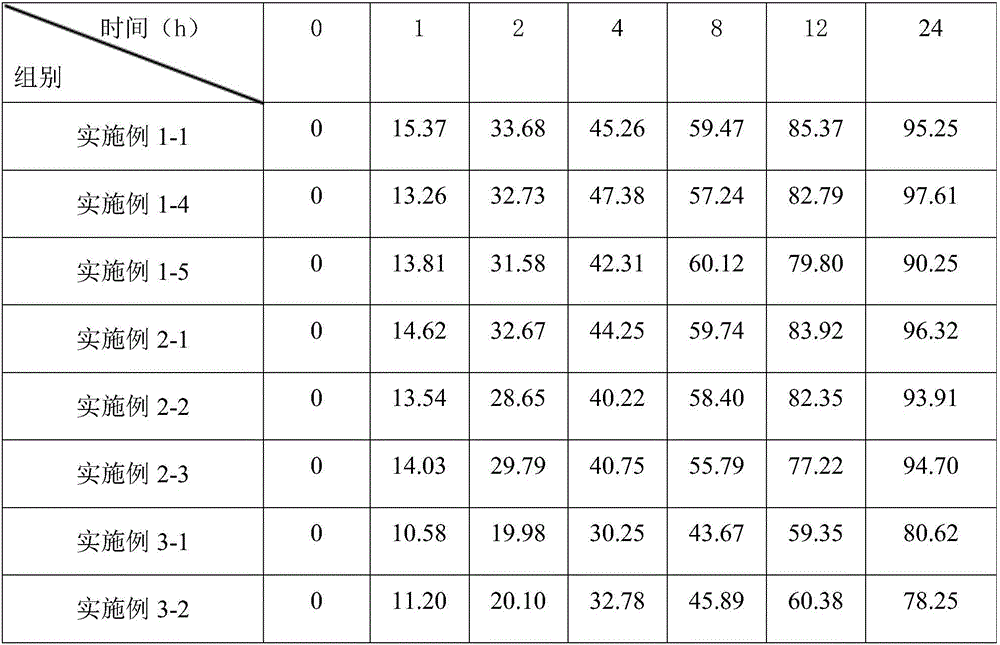
Embodiment 1-1
[0064] 1. Preparation of Haemophilus influenzae type B capsular saccharide
[0065] 1. Use CY medium to ferment the Hib strain at 37°C, inactivate the fermentation broth with formaldehyde, centrifuge to get the supernatant, and use cetyltrimethylammonium bromide (CTAB) for precipitation. Then use 0.5-1M NaCl to dissociate the complex precipitation, add glacial ethanol to a final concentration of 25% (v / v) to precipitate nucleic acid, then add glacial ethanol to a final concentration of 75% (v / v), and precipitate to obtain rough sugar .
[0066] 2. The above crude sugar was dissolved in sodium acetate solution, extracted 5-6 times with cold phenol, and finally concentrated by ultrafiltration to remove phenol residue, and refined sugar was obtained by 75% (v / v) ethanol precipitation, and stored at -20°C for later use.
[0067] 2. Preparation of pneumococcal polysaccharide
[0068] 1. Select seven serotypes (4, 6B, 9V, 14, 18C, 19F, 23F) of pneumococci for culture;
[0069]2. ...
Embodiment 1-2
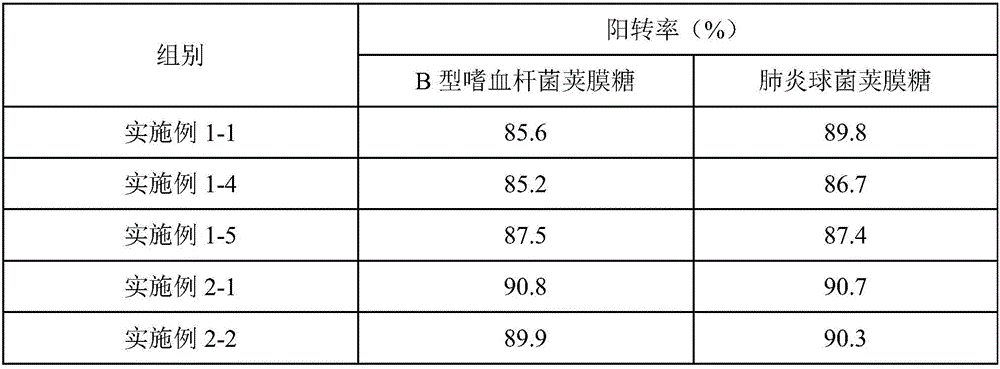
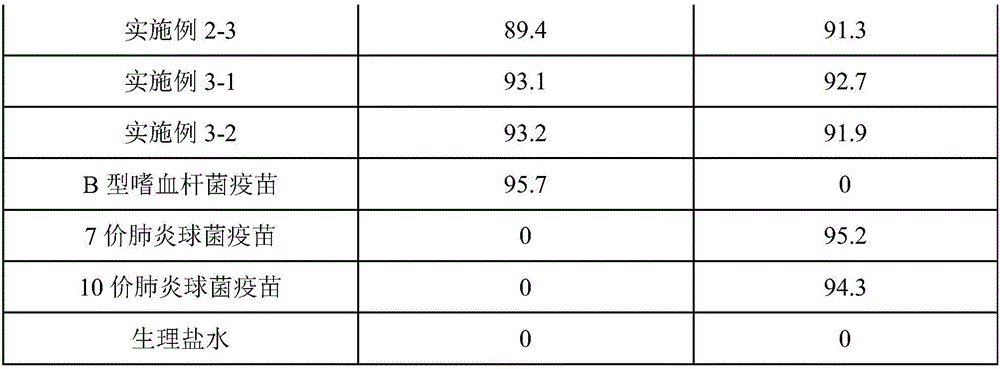
[0086] The only difference between this example and Example 1-1 is that the order of adding polysaccharides in this example is to add pneumococcal capsular saccharide and Haemophilus influenzae type B capsular saccharide at the same time. There is no chromatographic peak of Haemophilus influenzae type B capsular saccharide after the order is changed, which proves that adding pneumococcal capsular saccharide and Haemophilus influenzae type B capsular saccharide at the same time according to the original ratio will form a competitive relationship and affect the pod of Haemophilus influenzae type B. The normal conjugation process of membrane sugars, so the technical solution cannot be realized.
Embodiment 1-3
[0088] The only difference between this example and Example 1-1 is that the order of adding polysaccharides in this example is to add the capsular saccharide of pneumococcus first, and then add the capsular saccharide of Haemophilus influenzae type b. There is no chromatographic peak of capsular saccharide of Haemophilus influenzae type B after the order is changed, which proves that the addition of capsular saccharide of pneumococcus first and the capsular saccharide of Haemophilus influenzae type B according to the original ratio will form a competitive relationship and affect the haemophilus of type B influenza The normal conjugation process of bacillus capsular saccharide, therefore can't realize this technical scheme.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com