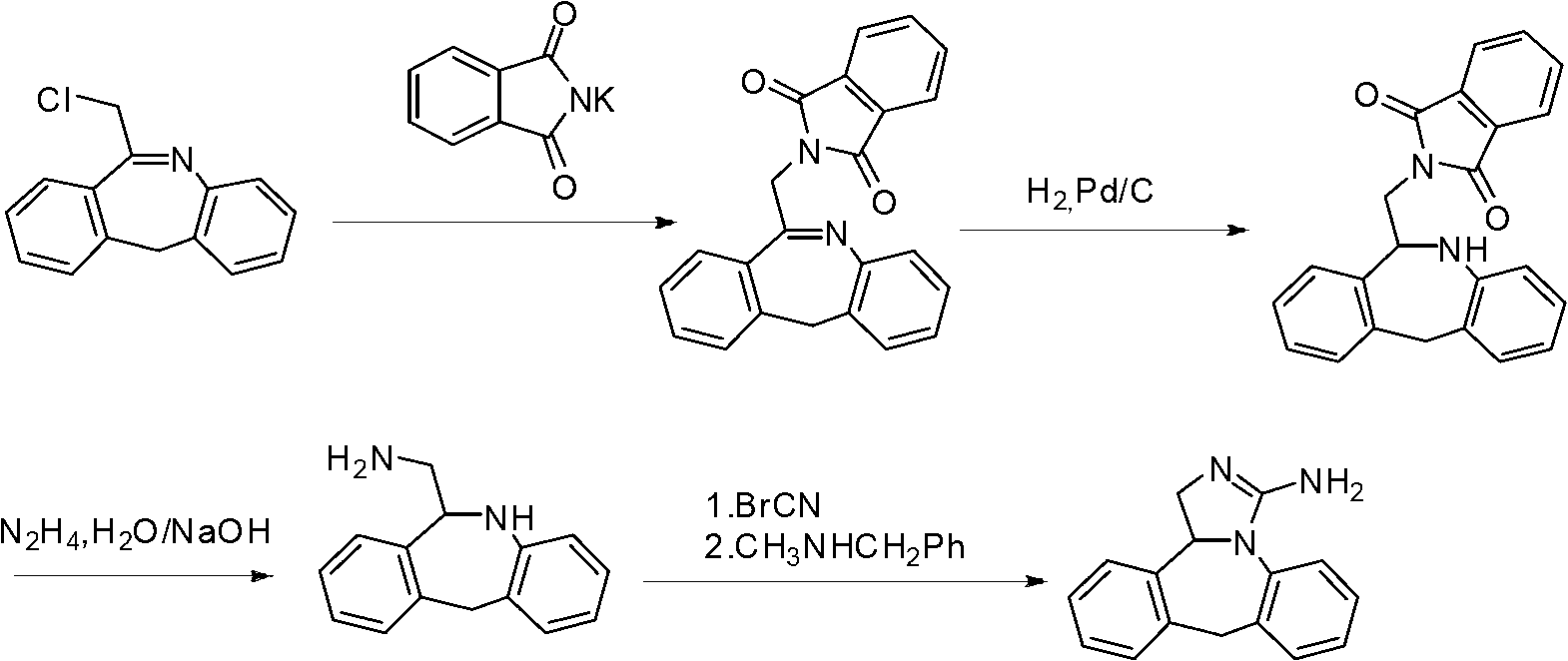
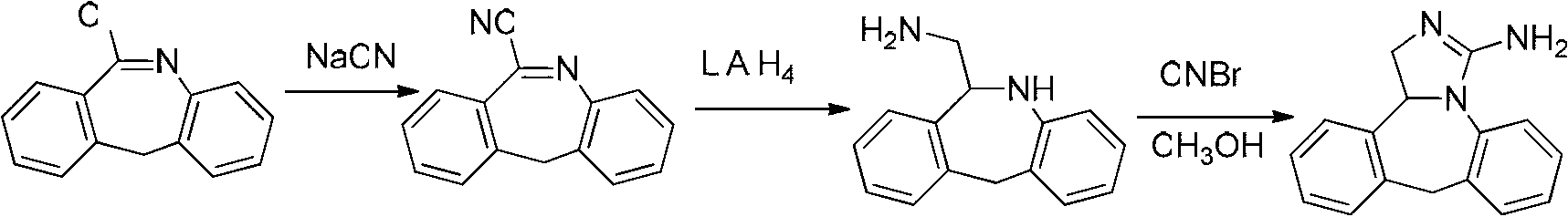
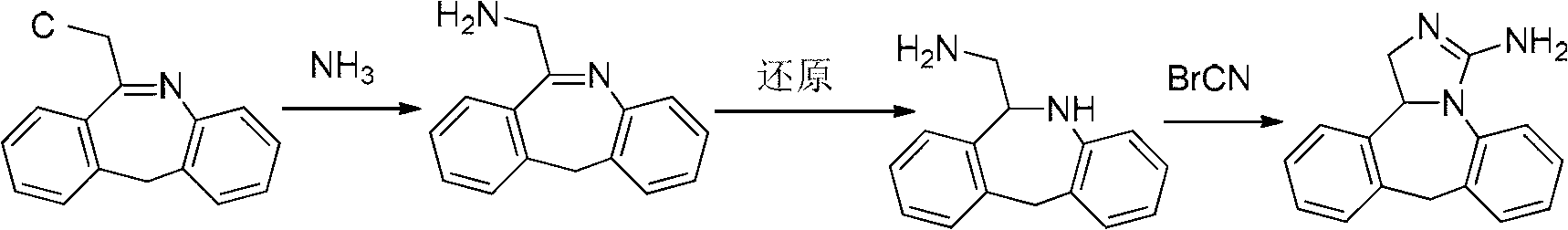
Synthesis method of epinastine
A synthetic method, the technology of epinastine, applied in the field of new chemical synthesis, can solve the problems of unsuitability for industrial production and low reaction yield, and achieve the effects of low cost, high product purity, and simple route process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthetic method of embodiment 1 epinastine
[0046] Include the following steps:
[0047] (1), 2-benzylaniline ( Synthesis of Intermediate 3)
[0048] Dissolve 2-aminobenzophenone I (6.0g, 30.45mmol, raw material 1) in 55mL of trifluoroacetic acid, slowly add triethylsilane (10.5g, 90.5mmol) dropwise at 0°C, and move to room temperature after 30min React for 8 hours until the reaction is complete. Thin-layer chromatography (TLC) detects the end point of the reaction, and the ratio shift value Rf=0.5 [developing solvent: V (petroleum ether) / V (ethyl acetate)=20 / 1]. Remove the solvent trifluoroacetic acid under reduced pressure, add saturated potassium carbonate aqueous solution and ethyl acetate to separate the liquid, and extract the aqueous phase with ethyl acetate, then combine the organic phase, wash the organic phase with water, dry, and concentrate to obtain 3.0 g of a light brown solid, which is Intermediate 3 is subject to the next reaction, and the yie...
Embodiment 2
[0068] The synthetic method of embodiment 2 epinastine
[0069] Include the following steps:
[0070] (1), (2-aminophenyl) (phenyl) methanol ( Synthesis of Intermediate 2)
[0071] Dissolve 2-aminobenzophenone I (6.0 g, 30.45 mmol, raw material 1) in 40 mL of ethanol, add sodium borohydride (2.31 g, 60.9 mmol) in portions at 0°C, and then move to room temperature for 3 h until The response is complete. The end point of the reaction was detected by thin-layer chromatography (TLC), and the ratio shift value Rf=0.3 [developing solvent: V (petroleum ether) / V (ethyl acetate)=10:1]. Add a small amount of water dropwise to remove the remaining sodium borohydride, spin off the solvent ethanol under reduced pressure, add water and ethyl acetate to separate the liquid, and extract the aqueous phase with ethyl acetate, then combine the organic phase, wash the organic phase with water, dry, and concentrate to obtain a light yellow 6.1 g of the solid is intermediate 2, and the next st...
Embodiment 3
[0088] The synthetic method of embodiment 3 epinastine
[0089] Include the following steps:
[0090] (1), 2-benzylaniline ( Synthesis of Intermediate 3)
[0091] Dissolve 2-aminobenzophenone I (6.0g, 30.45mmol, raw material 1) in 40mL of acetic acid, slowly add trimethylsilane (6.79g, 91.5mmol) dropwise at 0°C, and continue to react at 0°C for 30min Moved to 40 ° C conditions for 6h until the reaction is complete. Thin-layer chromatography (TLC) detects the end point of the reaction, and the ratio shift value Rf=0.5 [developing solvent: V (petroleum ether) / V (ethyl acetate)=20:1]. Spin off the solvent acetic acid under reduced pressure, add saturated potassium carbonate aqueous solution and ethyl acetate to separate the liquid, extract the aqueous phase with ethyl acetate, combine the organic phase, wash the organic phase with water, dry and concentrate to obtain 2.6 g of a light brown solid, which is the intermediate 3. The next reaction is to be carried out, and the yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com