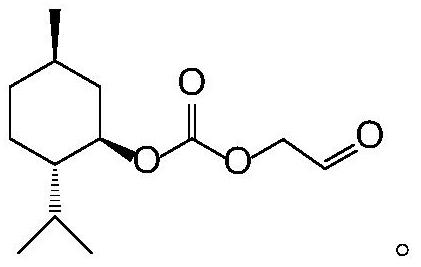
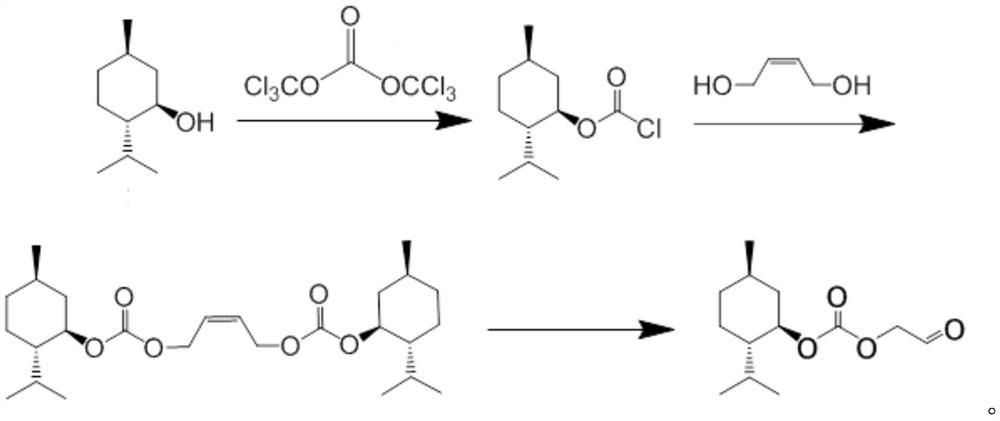
Synthesis method of acetaldehyde alcohol optical active ester
A technology of optical activity and synthesis method, which is applied in the field of drug synthesis, can solve the problems of low product yield, complex synthesis process, and low atom utilization rate, and achieve good reaction selectivity, simple synthesis process, and cheap and easy-to-obtain starting materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of synthetic method of acetaldehyde optically active ester, specifically comprises the following steps:
[0026] 1) Preparation of L-menthyl chloroformate: Add 20ml of dichloromethane into the reactor, place the instrument in a cold well, set the temperature to -10°C, and turn on the stirring paddle, then add 3.12g (0.02mol) of L-menth Alcohol, 2.376g (0.008mol) triphosgene, stir until completely dissolved, take the dropping funnel, add 1.896g (0.024mol) of pyridine and 40ml of dichloromethane and mix evenly, and slowly drop the solution into the reactor , the dropping rate is controlled at 1 drop per second, and the reaction is stirred for 4 hours to obtain a liquid phase containing L-menthyl chloroformate, and the second step of the reaction is ready to start after the reaction is determined to be complete by TLC detection;
[0027] 2) Preparation of bismenthyl carbonate: add the liquid phase containing L-menthyl chloroformate in the first step into the reactor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com