Synthesis method of ipratropium bromide
A technology of ipratropium bromide and its synthetic method, which is applied in the field of synthesis of ipratropium bromide, can solve the problems of inability to recycle methyl bromide and low yield, and achieve the effects of improving product quality, increasing conversion rate, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
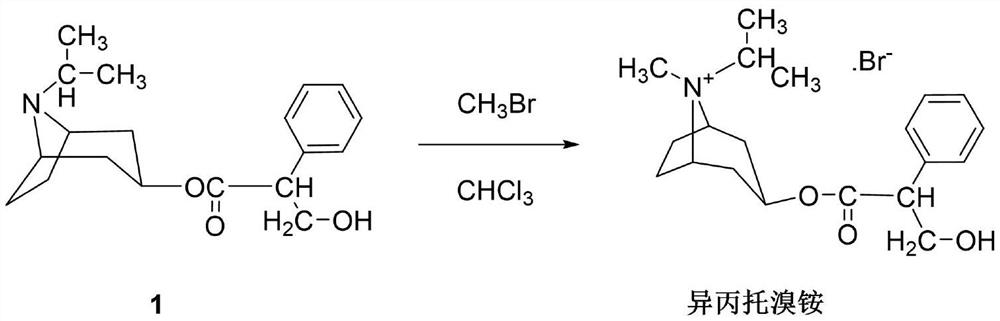
[0036] Put the intermediate 1 (2kg) and 24L of chloroform into the reaction tank to dissolve them all, cool to 0°C, and feed a total of 5kg of methyl bromide three times with an interval of 1 hour between each time, keeping the temperature at 0°C. After passing through, stir at 0°C for 16h, then raise the temperature to 25°C and stir for 4h. At the same time, during the stirring reaction, under the protection of nitrogen, it was cooled with a serpentine condenser at -25°C to prevent the loss of methyl bromide. Then raise the temperature to 40°C and stir for 4 hours, close the condenser, open the distillation device, and fully recover excess methyl bromide.
[0037] After recovery of methyl bromide, cool to room temperature, filter, soak the filter cake fully with chloroform, filter dry, and vacuum-dry at 70°C for 6 hours to obtain the crude product of ipratropium bromide, which is recrystallized in a mixed solvent of methanol and acetone, and washed with acetone , Vacuum dryi...
Embodiment 2
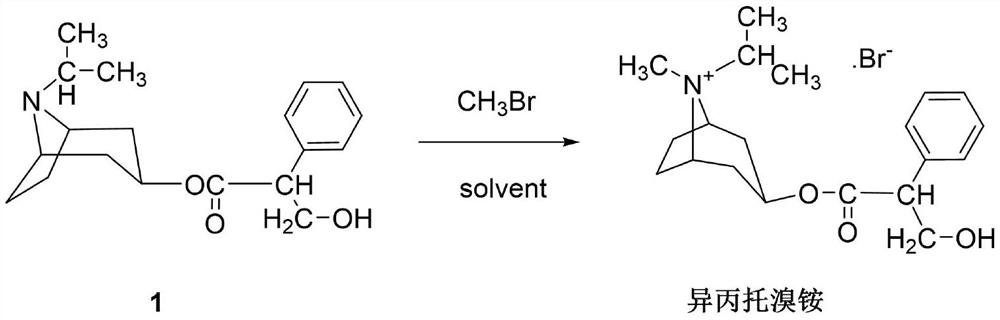
[0040] Put the intermediate 1 (2kg) and 24L of chloroform into the reaction tank to dissolve them all, cool to -5°C, and feed a total of 5.68kg of methyl bromide in three times, with an interval of 1.5h between each time, and keep the temperature at -5°C . After passing through, stir at -5°C for 17h, then raise the temperature to 27°C and stir for 5h. During the stirring reaction, under the protection of nitrogen, it was cooled with a serpentine condenser at -25°C to prevent the loss of methyl bromide. Then raise the temperature to 50°C and stir for 3 hours, close the condenser, open the distillation device, and fully recover excess methyl bromide.
[0041] After recovery of methyl bromide is complete, cool to room temperature, filter, soak the filter cake in chloroform, filter dry, and vacuum-dry at 75°C for 7 hours to obtain the crude product of ipratropium bromide, which is recrystallized from a mixed solvent of methanol and acetone, and washed with acetone. Vacuum drying...
Embodiment 3
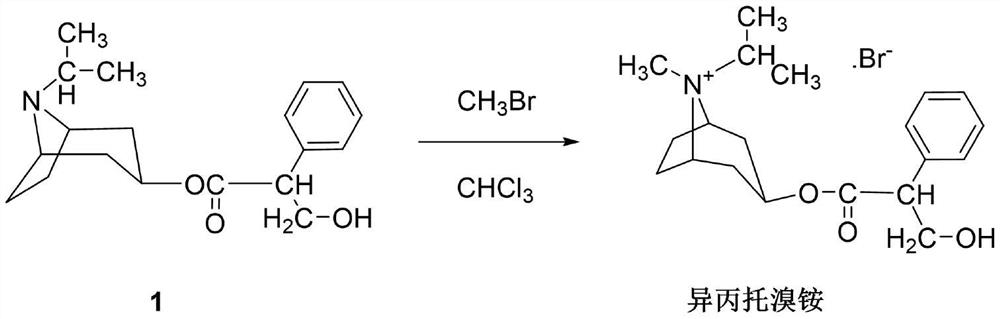
[0044] Put intermediate 1 (2.3kg) and 25L of chloroform into the reaction tank to dissolve them all, cool to -3°C, and feed a total of 4.81kg of methyl bromide in four times with an interval of 1 hour between each time, and keep the temperature at -3°C. ℃. After passing through, stir at -3°C for 16h, then raise the temperature to 23°C and stir for 4h. During the stirring reaction, under the protection of nitrogen, it was cooled with a serpentine condenser at -25°C to prevent the loss of methyl bromide. Then raise the temperature to 45°C and stir for 5 hours, close the condenser, open the distillation device, and fully recover excess methyl bromide.
[0045] After recovery of methyl bromide is complete, cool to room temperature, filter, soak the filter cake in chloroform, filter dry, and vacuum-dry at 75°C for 7 hours to obtain the crude product of ipratropium bromide, which is recrystallized from a mixed solvent of methanol and acetone, and washed with acetone. After vacuum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com