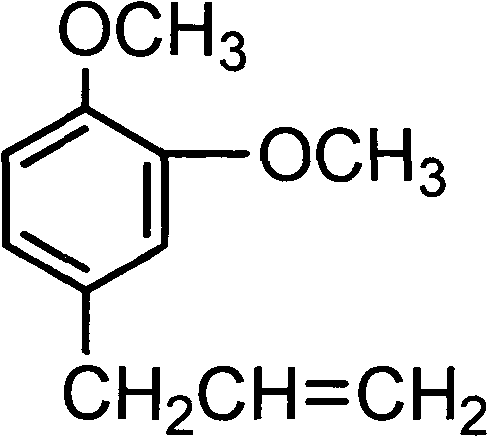
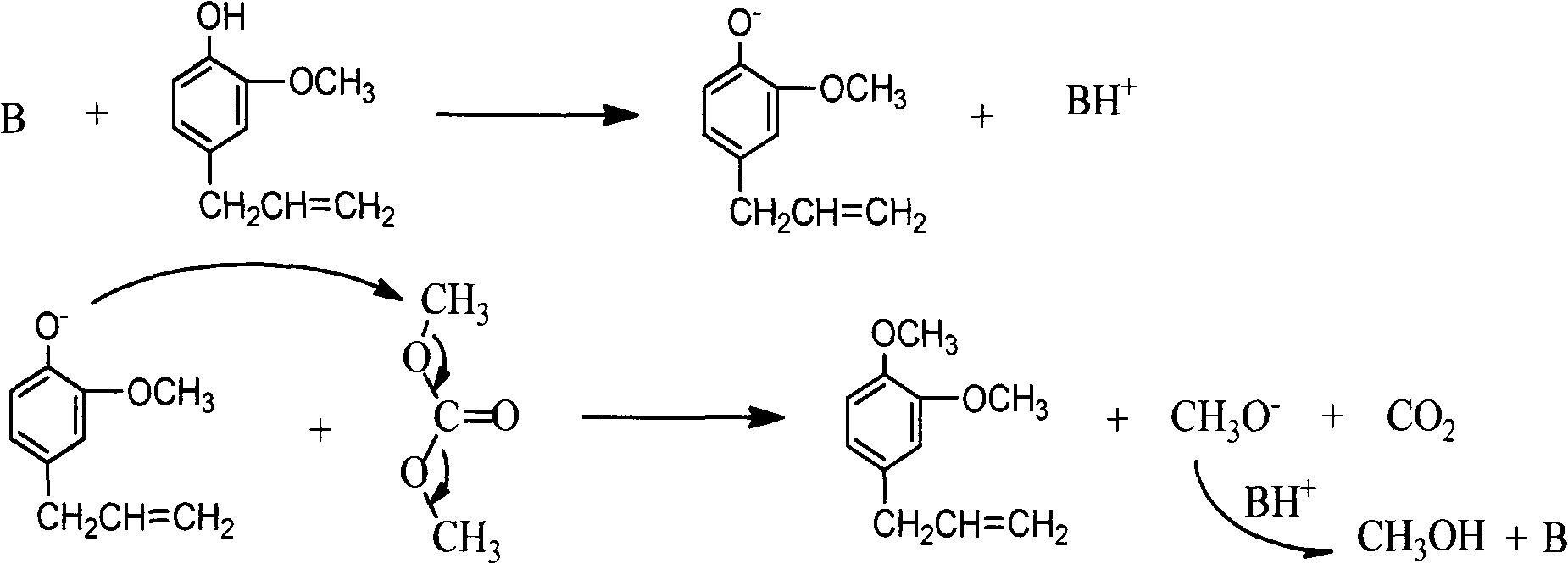
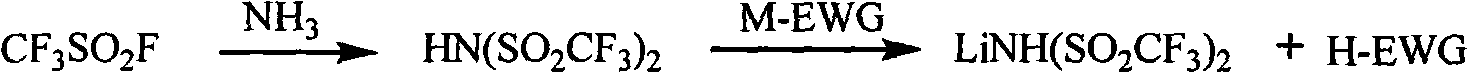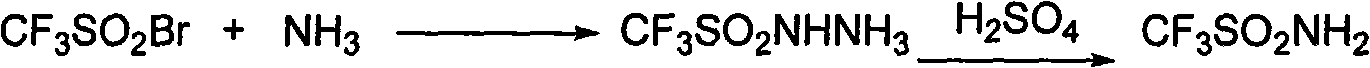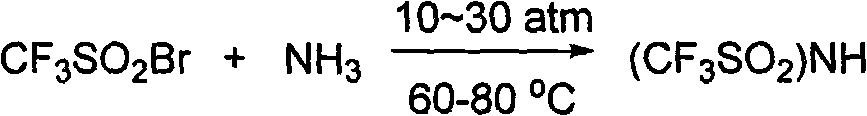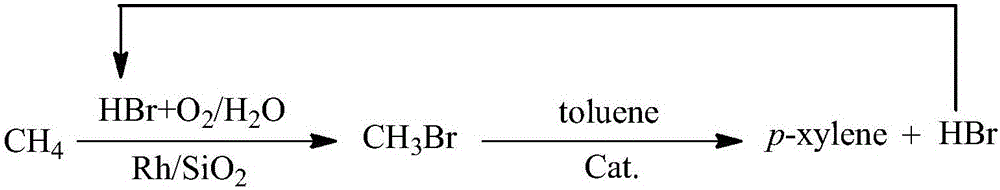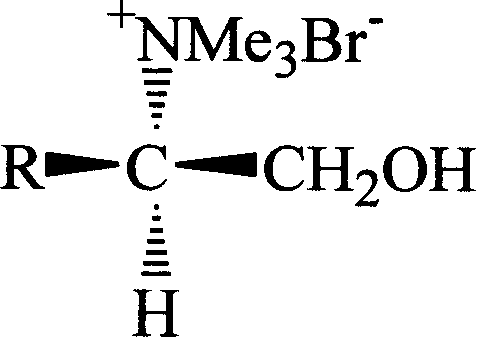Patents
Literature
124 results about "Bromide methyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula CH3Br. This colorless, odorless, nonflammable gas is produced both industrially and particularly biologically.
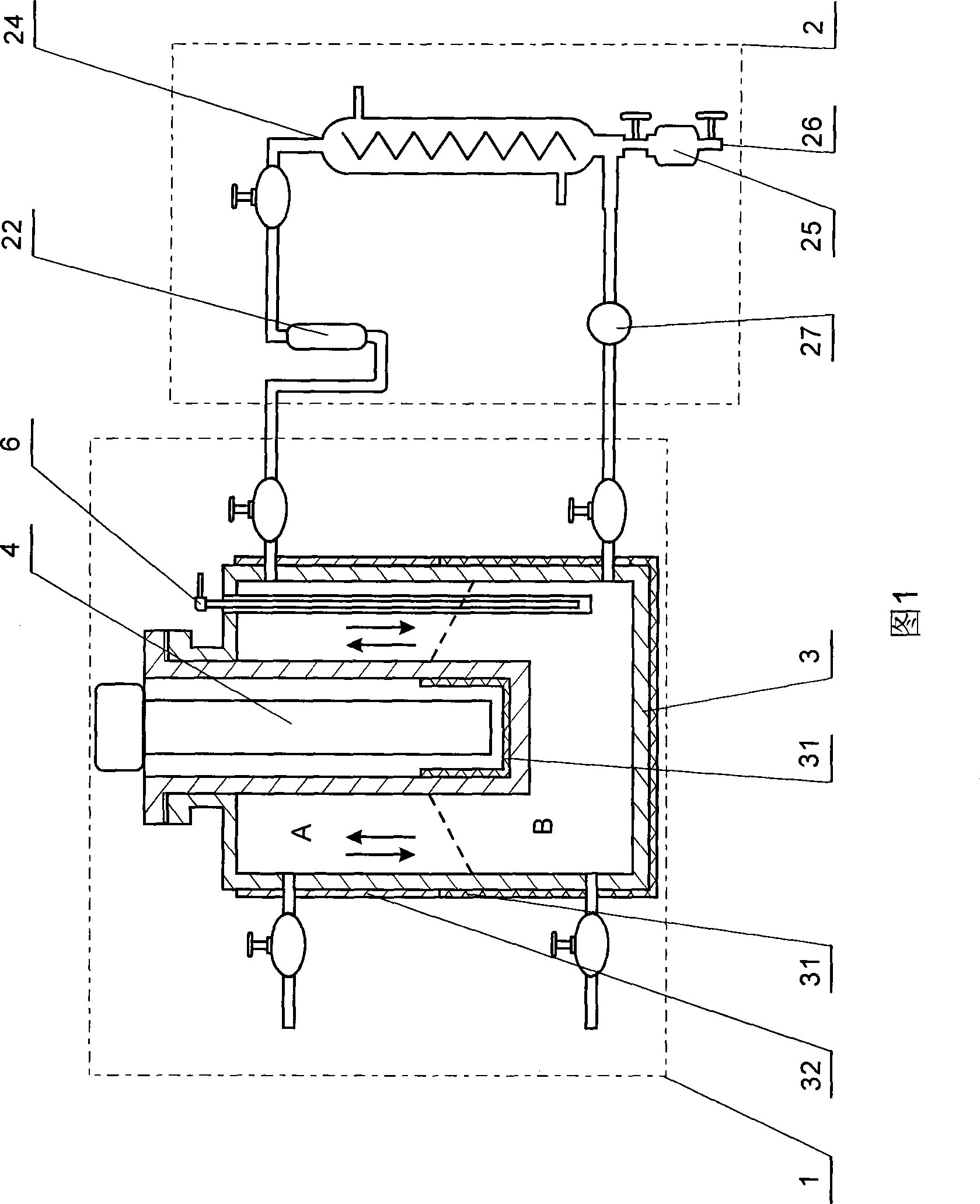
Structural fumigation process and apparatus
InactiveUS6047496AReduce the amount requiredNot extending current fumigant exposure timeFood preservationFumigatorsCombustible gasLiquid carbon
Processes and devices for producing a heated non-flammable gaseous fumigant for structural fumigation use by the application of a carrier gas, such as carbon dioxide (102), to a heater (103) either prior or subsequent to mixing the gas with a toxic agent gas, such as methyl bromide (213), such as by means of a mixer (214) and applying the mixture of gasses as a structural fumigant to eradicate target pests within a structure (106). In the preferred embodiment, the gaseous carbon dioxide is formed by flashing liquid carbon dioxide (614) directly to the gaseous state in a heater (630) prior to being mixed with methyl bromide (654) in a mixer (648) and applying the mixture of gasses as a structural fumigant within a structure (668).
Owner:LEITNER KENNETH D +1
Synthesis process of vecuronium bromide
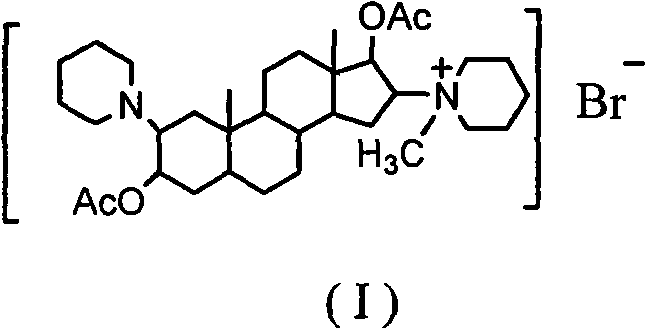
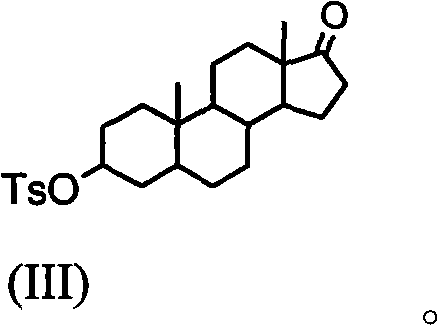
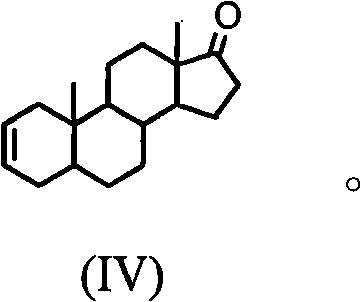
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
Hyperbranched polyamide modified chitosan quaternary ammonium salt microsphere for wastewater treatment and preparation method and application thereof
ActiveCN105669986ALarge specific surface areaImprove adsorption capacityOther chemical processesWater contaminantsEthylenediamineQuaternary ammonium cation
The invention provides hyperbranched polyamide modified chitosan quaternary ammonium salt which is obtained through the reaction of hyperbranched polyamide, chitosan and alkyl halide.The hyperbranched polyamide is prepared from trimesoyl chloride and ethylenediamine through a solution polymerization method.The alkyl halide comprises methyl iodide, ethyl iodide, methyl bromide or ethyl bromide.The microsphere prepared from the quaternary ammonium salt is applied to treatment of methyl orange wastewater, the final dye removal rate can reach 99.67%, the microsphere is renewable, and the preparation process is simple and free of pollution.
Owner:ZHANJIANG JIALI GLOVE PRODS
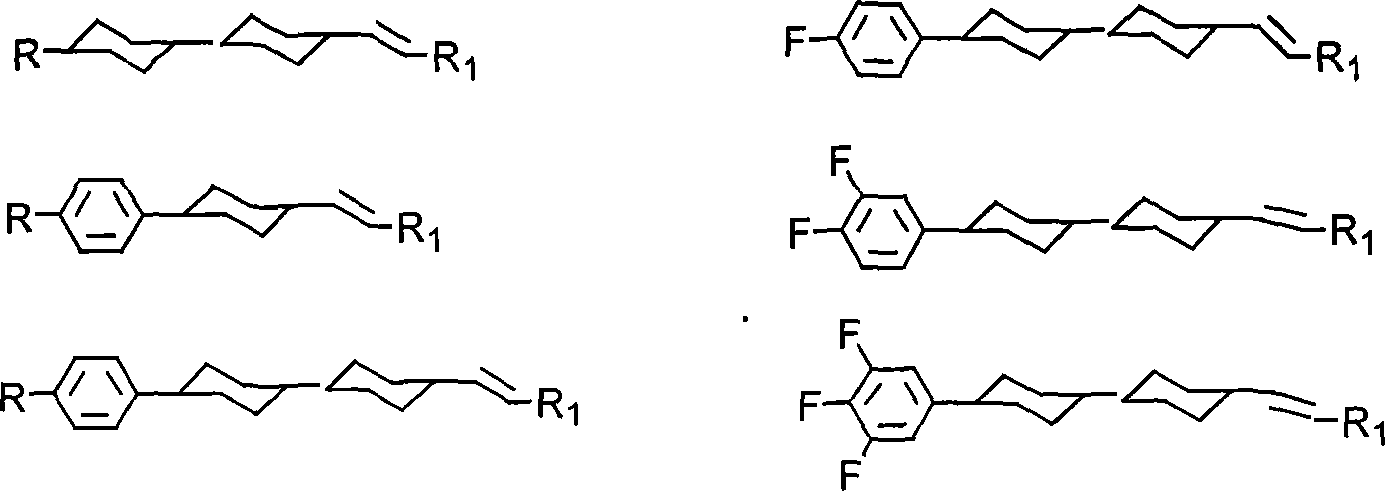
Method for producing cyclohexyl group olefin hydrocarbon liquid crystal material
ActiveCN101244977ASmall optical anisotropyLow viscosityLiquid crystal compositionsHydrocarbonsAlkanePhosphonium
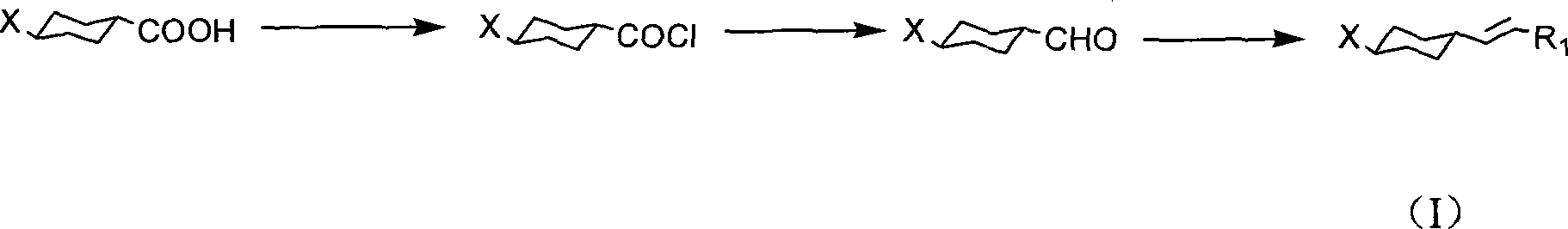
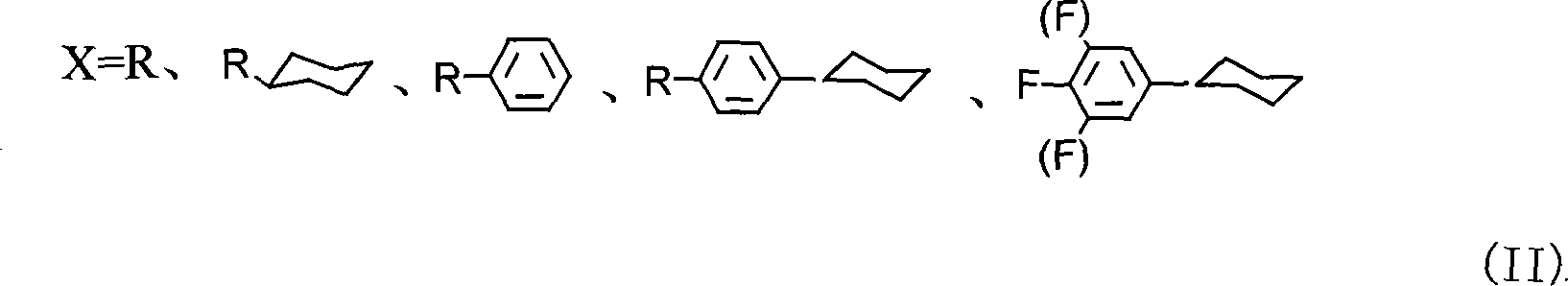
The invention discloses a preparation method of trans-4-replacing cyclohexyl formaldehyde and trans-4-replacing cyclohexyl olefin, which uses trans-4-replacing cyclohexyl formic acid as raw material, and prepares the trans-4-cyclohexyl olefin by acyl chloride, silicon hydrogen alkane reduction, and reaction with methyl bromide triphenylphosphine quarter phosphonium. The preparation method of the trans-4-replacing cyclohexyl formaldehyde and the trans-4-replacing cyclohexyl olefin is simple in method, clean in using, and has wide application prospect.
Owner:内蒙古永太化学有限公司
Method for preparing sulfimide compound
InactiveCN101983960AHigh boiling pointEasy to operateSulfonic acid amide preparationBromotrifluoromethaneOrganic synthesis
The invention provides a method for preparing a sulfimide compound as shown in the formula (1): MN(SO2CF3) (SO2CF3), wherein M represents H and IA group alkali metals. The sulfimide compound utilizes bromotrifluoromethane easily obtained in industrial production as a raw material, and is prepared by the steps of sulfinatodehalogenation, bromination and ammoniation. The method can be used for organic synthesis, has high purity and yield as well as low cost, and can solve the problem that the sulfimide compound is difficult to realize large-scale application in industrial production due to high cost.
Owner:SHANGHAI SINOFLUORO SCI
Azeotrope-like compositions of 1-chloro-3,3,3-trifluoropropene and methyl iodide
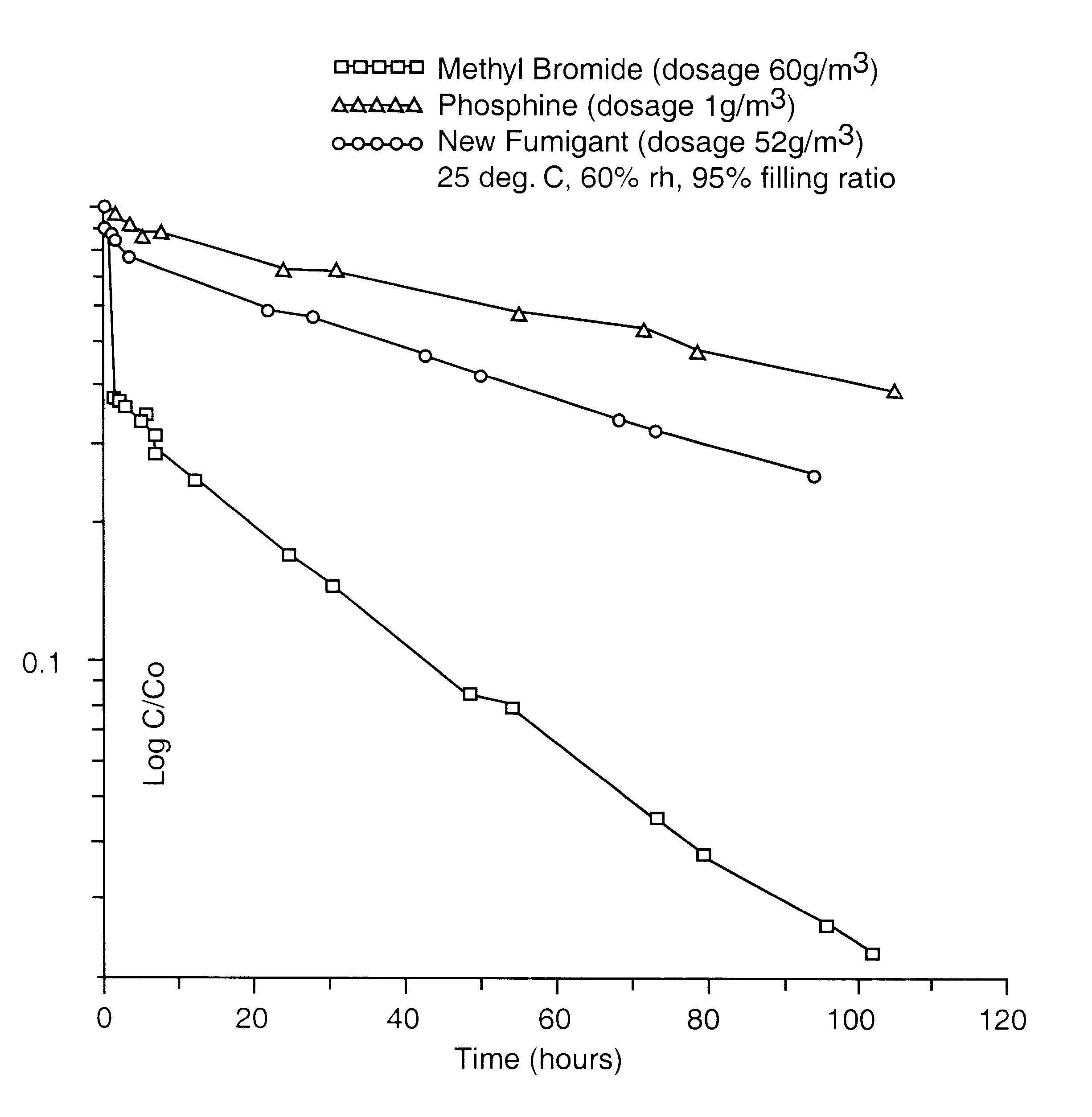
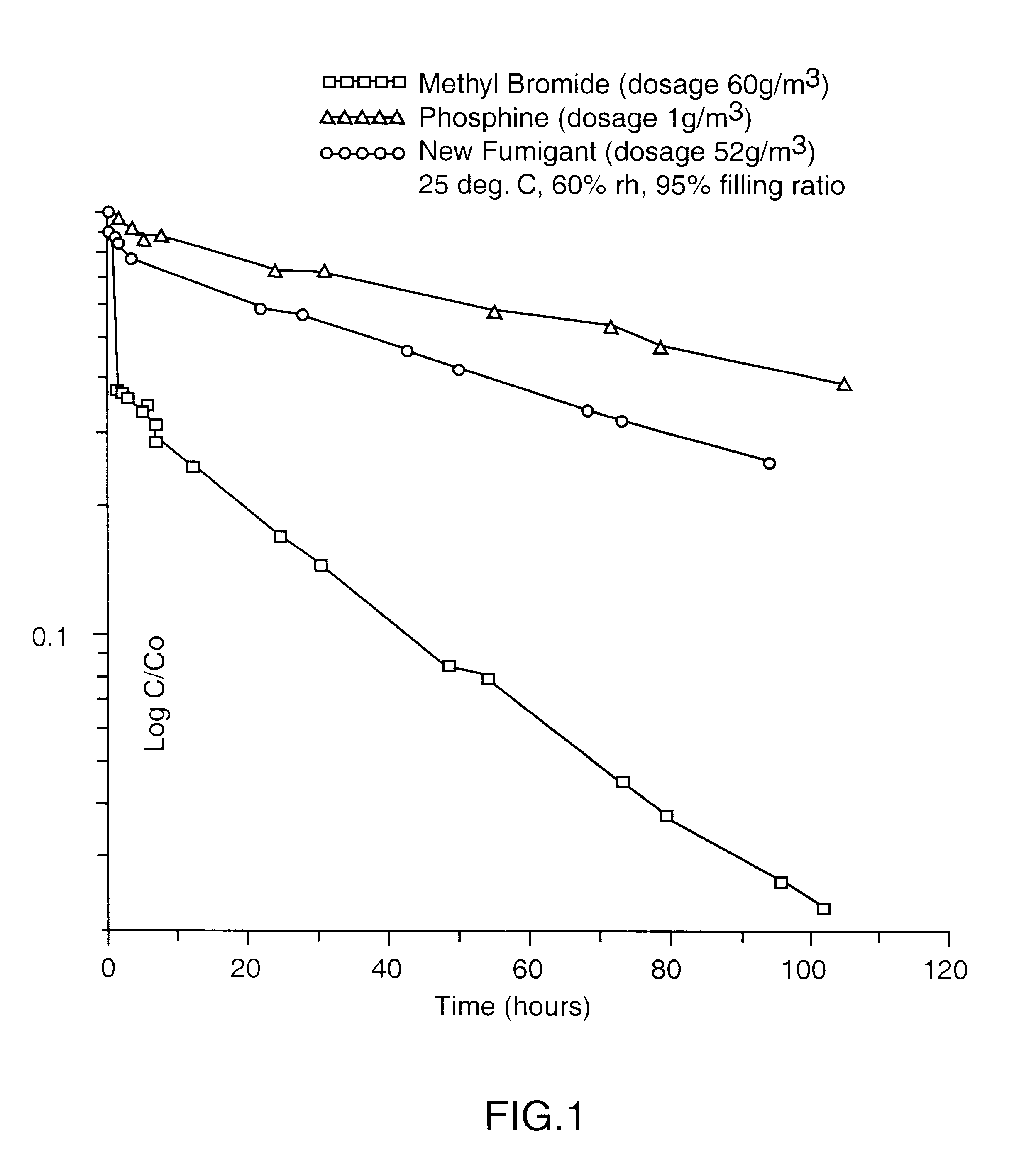
An azeotropic or azeotrope-like composition comprising a mixture of methyl iodide, 1-chloro-3,3,3,-trifluoropropene, and optionally one or more of fluorocarbons and / or hydrofluorocarbons. The compositions are present as a gas, at temperatures of about 30° C. or below. The inventive compositions serve as a non-ozone-depleting gaseous fumigant which is useful in a variety of applications, in place of methyl bromide. These compositions serve as a drop-in replacement for gaseous methyl bromide, providing the benefits of a methyl iodide fumigant while also utilizing existing methyl bromide equipment.
Owner:HONEYWELL INT INC
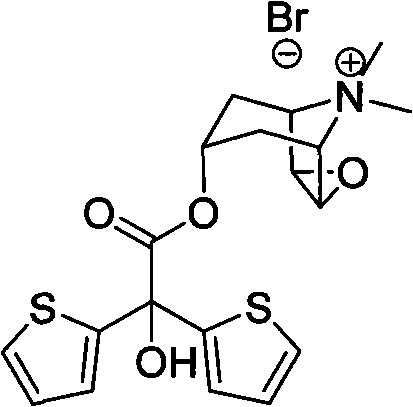
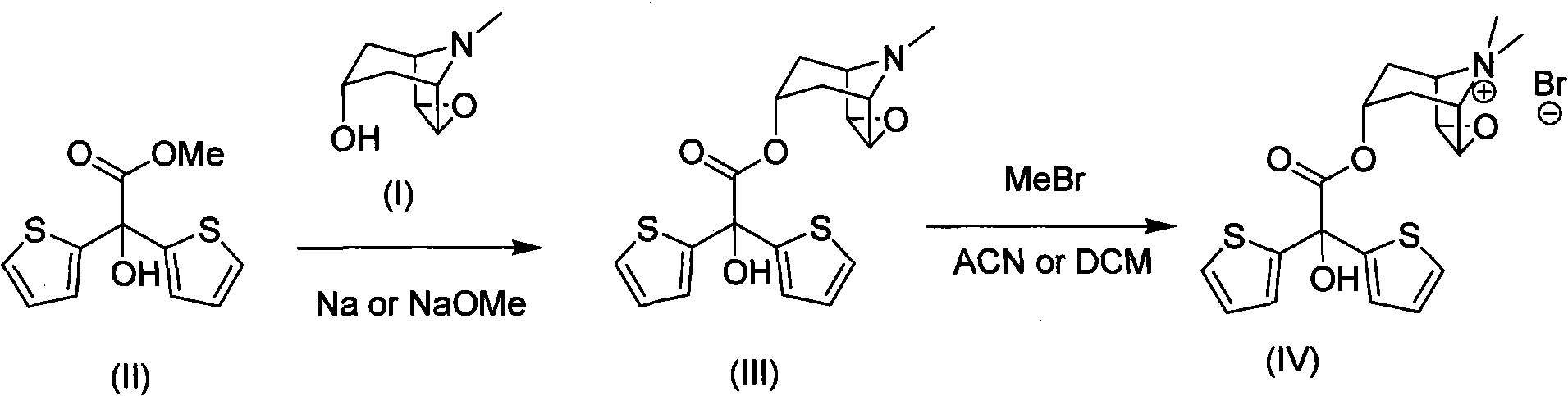
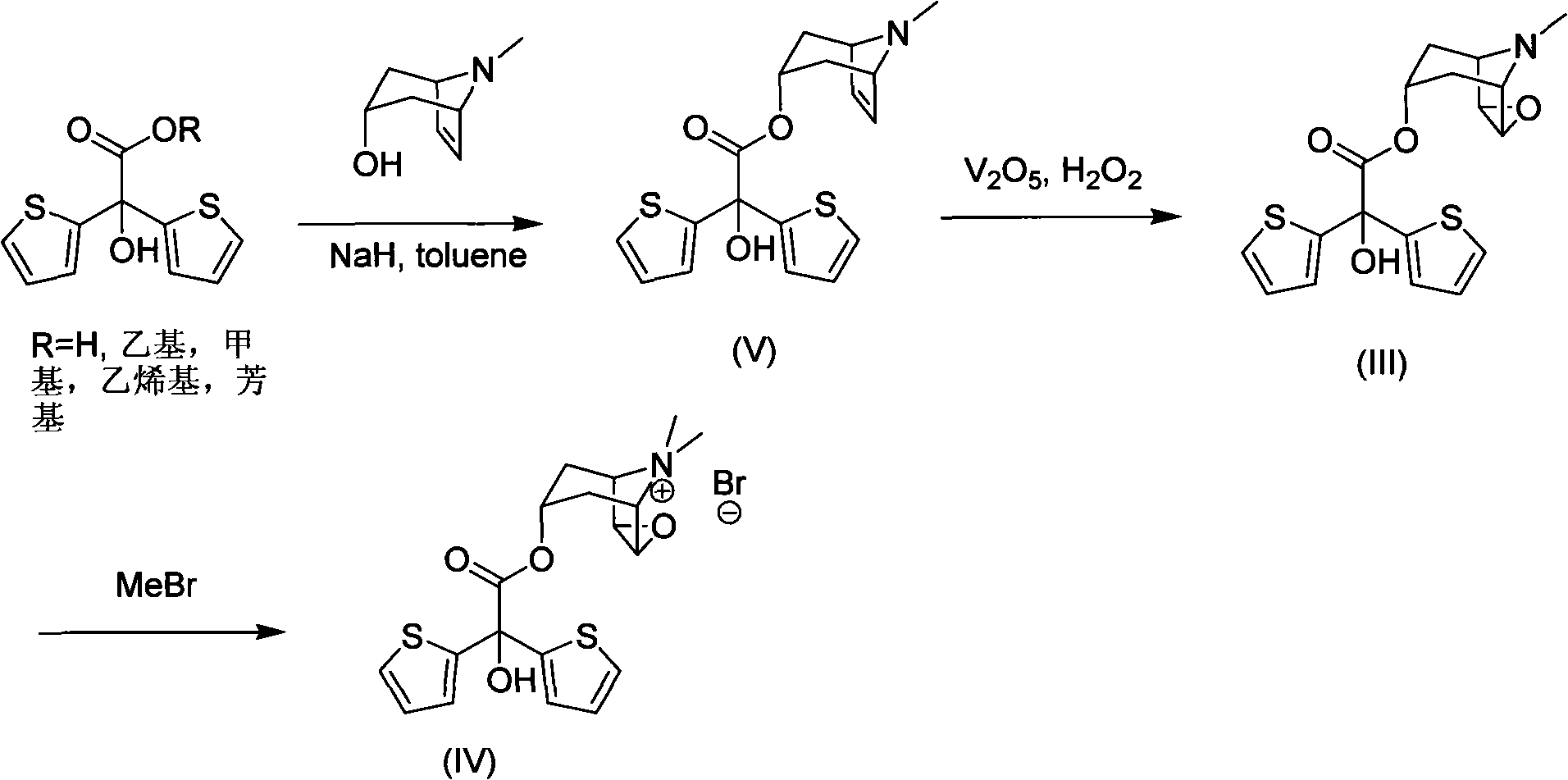
Method for preparing tiotropium bromide
The invention discloses a method for preparing tiotropium bromide, which comprises the following steps of: adding scopine, bis(2-thiophene) methylglycollate and potassium carbonate into an organic solvent, and heating and refluxing the mixture for 1 to 4 hours at the temperature of between 120 and 150 DEG C under inert atmosphere to prepare bis(2-thiophene) scopine glycolate; and adding the bis(2-thiophene) scopine glycolate and methyl bromide into the organic solvent, and reacting the mixture for 15 to 25 hours at the room temperature under the inert atmosphere with stirring to prepare the tiotropium bromide. Compared with the prior art, the method for preparing the tiotropium bromide has the advantages of high yield, easy operation, mild condition, low environmental pollution, low cost and large-scale production.
Owner:NANJING JINDANCHENG MEDICINETECH
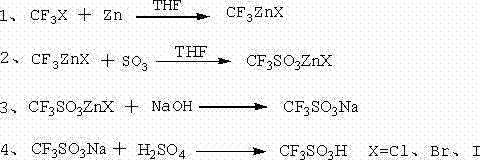
Trifluoromethanesulfonic acid preparation method
ActiveCN104725283AHigh yieldAvoid it happening againSulfonic acid preparationDistillationTriflic acid
Owner:JIANGXI GUOHUA IND CO LTD
Method for preparing tiotropium bromide
The invention discloses a method for preparing tiotropium bromide, which comprises the following steps of: preparing scopine-2,2-dithienyl glycolate from scopine and methyl 2,2-dithienyl glycolate, reacting the scopine-2,2-dithienyl glycolate with methyl bromide to prepare a tiotropium bromide crude product, and refining the tiotropium bromide crude product to obtain a tiotropium bromide finishedproduct. The method is characterized in that: the scopine and the methyl 2,2-dithienyl glycolate undergo ester exchange reaction under the action of dimethylbenzene, and the mixed catalysts of sodiumand sodium methoxide, and after the reaction, reaction solution is post-treated to obtain the scopine-2,2-dithienyl glycolate. In the method, in the process of preparing the scopine-2,2-dithienyl glycolate, the sodium and the sodium methoxide are simultaneously taken as the catalysts, and scopine isomer content after the reaction is less than 0.1 percent which completely meets specifications in the trial standards of European pharmacopoeia; and the method solves a big problem for the conventional preparation of the tiotropium bromide and is easy to realize industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
Carbonyl sulphide insecticide
The gaseous chemical compound, carbonyl sulphide, has hitherto been unknown as a fumigant for the control of insects and mites. Experiments have shown conclusively that carbonyl sulphide can be used as such a fumigant, with fumigation properties comparable to those of phosphine and methyl bromide. The effectiveness of carbonyl sulphide against insects (both adult and immature stages), mites, termites and moulds is demonstrated. In addition, its low absorbtion by grain, lower flammability than phosphine, lack of influence on seed germination, and apparent environmental safety make carbonyl sulphide particularly beneficial as a fumigant of stored grain. It may also be used to fumigate other stored produce (including perishable foodstuff), soil, timber and spaces (such as buildings) and any material likely to be infested by insects or mites, or act as a source of such infestation.
Owner:COMMONWEALTH SCI & IND RES ORG
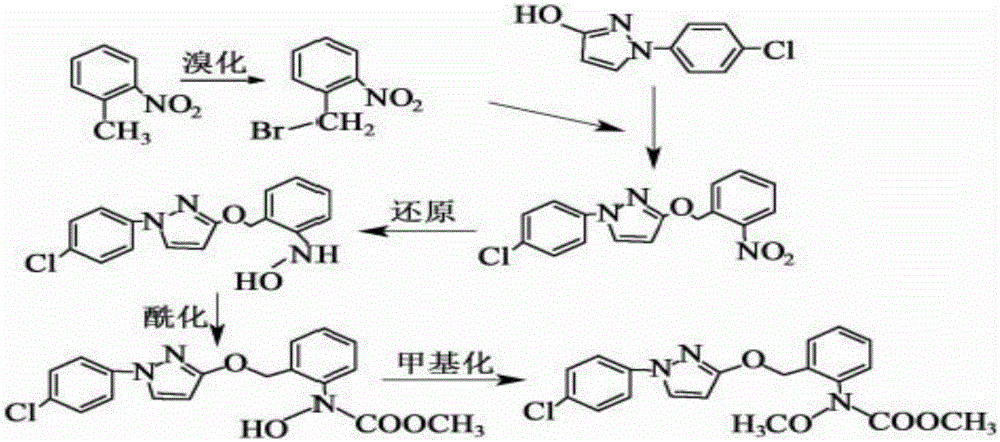
Synthesis technology of pyraclostrobin
InactiveCN106008347AImprove selective reducibilityHigh yieldOrganic chemistryMicro nanoHydroxylamine
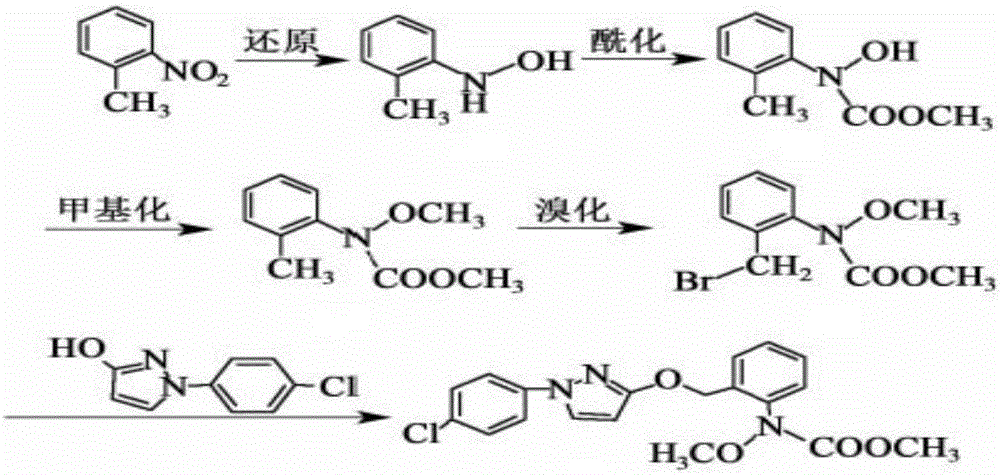
The invention provides a synthesis process of pyraclostrobin, which can be divided into five steps: (1) o-nitrotoluene and NH 4 Cl reduction reaction occurs under the catalysis of zinc powder and alloy micronano powder; (2) acylation reaction of hydroxylamine; (3) methylation reaction; (4) N-methoxy-N -2-bromomethylphenyl methyl carbamate; (5) use DMF as a solvent to dissolve N-methoxy-N-2-bromomethyl phenyl methyl carbamate and make a solution for subsequent use, and 1-( 4‑chlorophenyl)‑pyrazolol, K 2 CO 3 , acetone and put them into the reactor together, after heating up and refluxing, slowly add N-methoxy-N-2-bromomethylphenyl carbamate solution in the reactor, and the product pyrazole ether is obtained after the reflux reaction is completed Strostrobin. Compared with the prior art, the preparation method of the present invention is simple, the raw materials are cheap and easy to obtain, the reaction conditions are mild, and the purity and yield of the obtained target product are high.
Owner:ANHUI GUANGXIN AGROCHEM
Preparation method of micron-sized HZSM-5 molecular sieve and application of micron-sized HZSM-5 molecular sieve
InactiveCN106629769AExtended Diffusion PathEasy to prepareMolecular sieve catalystsHydrocarbon from halogen organic compoundsMolecular sieveStrong acids
The invention discloses a micron-sized HZSM-5 molecular sieve catalyst. According to the micron-sized HZSM-5 molecular sieve catalyst disclosed by the invention, the micron-sized HZSM-5 molecular sieve is designed and synthesized by utilizing double-template, and thus, not only can acidity of the surface of a zeolite catalyst be modulated but also a diffusion path of a product can grow by increasing a particle size, and then, the goal of increasing para-selectivity is achieved. The invention further provides a preparation method of the micron-sized HZSM-5 molecular sieve and catalytic application of the micron-sized HZSM-5 molecular sieve. The micron-sized HZSM-5 molecular sieve catalyst provided by the invention is synthesized by a double-template one-step hydrothermal method, so that the preparation process is simple and convenient; when being applied to methyl bromide methylated methylbenzene to prepare paraxylene, the molecular sieve shows higher catalytic activity and selectivity during reaction; the catalyst shows the higher selectivity, which dues to fewer strong acid sites and a larger grain size; moreover, the catalyst has no corrosion on a fixed bed reactor, and generated hydrogen bromide tail gas is easy to recycle, so that the catalyst belongs to an environmentally friendly catalyst; and after being deactivated, the catalyst can be repeatedly used for more than once through simple roasting.
Owner:HUNAN UNIV
Synthesis method of eugenol methyl ether
ActiveCN102351663ALess corrosiveEasy post-processingEther preparation by ester reactionsMethylating AgentMethyl carbonate
The invention discloses a synthesis method of eugenol methyl ether. In the method, eugenol and dimethyl carbonate (DMC) are taken as raw materials, and eugenol methyl ether is prepared through liquid-solid phase reaction under pressurization while base catalysis is performed. The green organic chemical raw material, namely DMC, is utilized for replacing traditional dimethyl sulfate, bromomethane, phosgene and other toxic and harmful methylating agents, and solid base is selected as a catalytic system. The synthesis method is a clean synthesis route of the eugenol methyl ether, is environmentally friendly, economic in preparation, convenient in post-treatment and low in corrosion to equipment. The method has great industrial application prospects.
Owner:黄山市巨龙生物能源科技有限公司
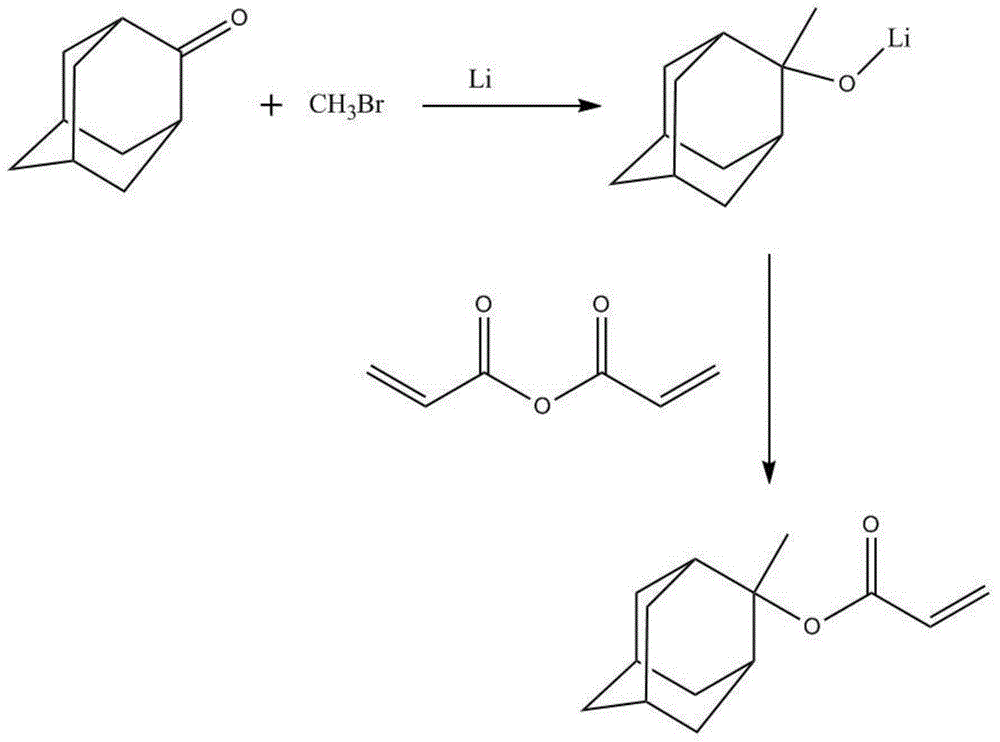
Preparation method of 2-methyl-2-adamantyl acrylate
ActiveCN104628557AOrganic compound preparationCarboxylic acid esters preparationState of artLithium metal
The invention provides a preparation method of 2-methyl-2-adamantyl acrylate, which is characterized in that under low temperature condition, an active intermediate is formed by adamantane ketone and bromomethane under effect of lithium metal, and the active intermediate and acrylic anhydride are reacted to obtain 2-methyl-2-adamantyl acrylate. In the provided synthetic method, through the selection of reaction condition of each step, such as reaction temperature, reaction proportion, reaction time and reaction raw materials, and the problems of difficult purification, high cost, low reaction yield, low purity of product and inapplicable large scale industrial production in prior art can be overcome.
Owner:XUZHOU B&C CHEM CO LTD
PTA (purified terephthalio acid) oxidized tail gas catalytic combustion processing method with low energy consumption
ActiveCN104566405AOperating pressure constantReduce consumptionIncinerator apparatusAir quality improvementEmission standardCatalytic burner
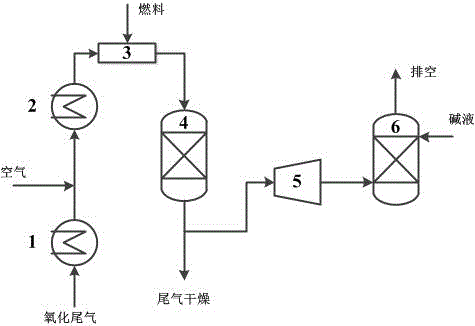
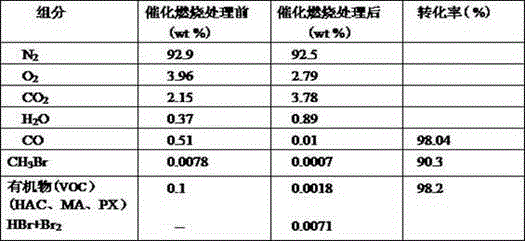
The invention discloses a PTA (purified terephthalio acid) oxidized tail gas catalytic combustion processing method with low energy consumption. Oxidized tail gas from a high-pressure absorbing tower enters a catalytic combustion pre-heater and is heated to 295-300 DEG C, and then is mixed fully with assistant fuel methanol and fed into a combustion reactor, wherein the addition of methanol is 6.5-7.0kg / t PTA, the operation pressure of the catalyst combustor is 1.0-1.2MPa, the gas inlet temperature is 295-300 DEG C, and the normal outlet temperature is 395-405 DEG C. Through catalytic combustion reaction, a small amount of organic matter and CO in the oxidized tail gas is combusted to generate CO2 and H2O, and methyl bromide is converted to bromide and hydrogen bromide. Most part of gas from the catalyst combustor enters into a tail gas expanding machine to act to recover energy. The gas inlet temperature of the tail gas expanding machine is 395-405 DEG C, the inlet pressure is 1.0-1.2MPa, the gas outlet temperature is 105-115 DEG C and the outlet pressure is 0.1MPa. The tail gas outputted from the expanding machine is washed with alkali by a tail gas washing tower to remove HBr and Br2, and then is exhausted into air after reaching an environmental protection emission standard. Compared with an existing HPCCU technology, the mechanical consumption amount is reduced by 6.5-7.0kg / tPTA, the methanol consumption amount is reduced by about 50%, the energy consumption of the catalytic combustion system is reduced significantly, the processed tail gas reaches the environment emission standard, and the economic benefits are significant.
Owner:NANJING POLYTECHNIC INSITUTE
Supported catalyst with mesoporous structure, and preparation method and application thereof
ActiveCN106140218AHigh selectivityEasy to industrializePhysical/chemical process catalystsHydrocarbon from halogen organic compoundsZincMetal
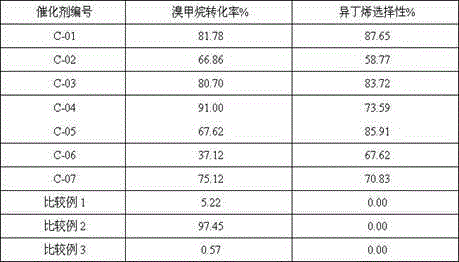
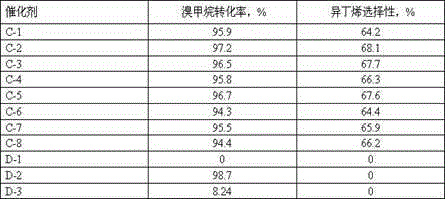
The invention discloses a supported catalyst with a mesoporous structure, and a preparation method and application thereof. The catalyst comprises, by weight, 0.5 to 20% of zinc oxide, 10 to 50% of zinc halide and 0.1 to 20% of a modification aid, with the balance being a modified mesoporous alumina carrier. The preparation method for the catalyst comprises the following steps: (1) preparing the modified mesoporous alumina carrier; (2) loading an active metal zinc precursor on the carrier obtained in the step (1) by using an equivalent-volume impregnation manner and carrying out drying and roasting so as to prepare a catalyst precursor; and (3) subjecting the catalyst precursor prepared in the step (2) to halogenation so as to prepare the catalyst for preparation of isobutene from halogenated methane. The catalyst is capable of obviously improving selectivity of isobutene when used for a reaction for preparation of isobutene from bromomethane.
Owner:CHINA PETROLEUM & CHEM CORP +1
Supported catalyst using modified ZSM-5 molecular sieve as carrier and preparation method and application thereof
ActiveCN106140259AHigh selectivityEasy to prepareMolecular sieve catalystsHydrocarbon from halogen organic compoundsHydrogenWater vapor
The invention discloses a catalyst using a modified ZSM-5 molecular sieve as a carrier and a preparation method and application thereof. The preparation method of the catalyst includes the following steps that 1, a modified hydrogen type ZSM-5 molecular sieve carrier is obtained by using water vapor and an acid treated hydrogen type ZSM-5 molecular sieve having complexing properties; 2, zinc oxide and / or an additive oxide are introduced to the modified hydrogen type ZSM-5 molecular sieve carrier; 3, incomplete bromination treatment is conducted on the modified hydrogen type ZSM-5 molecular sieve carrier introduced with the zinc oxide. The catalyst prepared by adopting the method is used for methyl bromide to prepare isobutylene, and the selectivity of isobutene can be remarkably improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
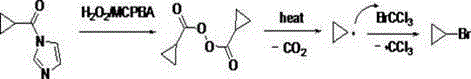
Novel method for synthesizing cyclopropyl bromide
ActiveCN104892355AEfficient synthesisAvoid pollutionHalogenated hydrocarbon preparationAcyl groupCarboxylic acid
The invention relates to a novel method for synthesizing cyclopropyl bromide. The novel method comprises the following steps: carrying out a reaction between cyclopropyl carboxylic acid and carbonyl diimidazole to generate cyclopropyl acyl imidazole, then mixing the intermediate with bromo-trichloromethane, adding an oxidizing agent slowly under the condition that the temperature is kept to be 10 to 140 DEG C for a free radical deacidification bromination reaction, so as to obtain a cyclopropyl bromide crude product, and carrying out simple normal pressure rectification to obtain a product of which the purity is 99 percent or higher. The novel method has the advantages that the reaction conditions are mild; the used reagents are conventional, cheap and high in availability; mercuric oxide, which is a highly toxic product, is not used any more; the novel method is suitable for industrial scale-up production.
Owner:CANGZHOU PURUI DONGFANG SCI & TECH +2
Novel chiral amino acid derivative and its synthetic method and use
InactiveCN1616411AEasy to splitSimple methodOrganic compound preparationCarboxylic compound preparationAcid derivativeKetoprofen
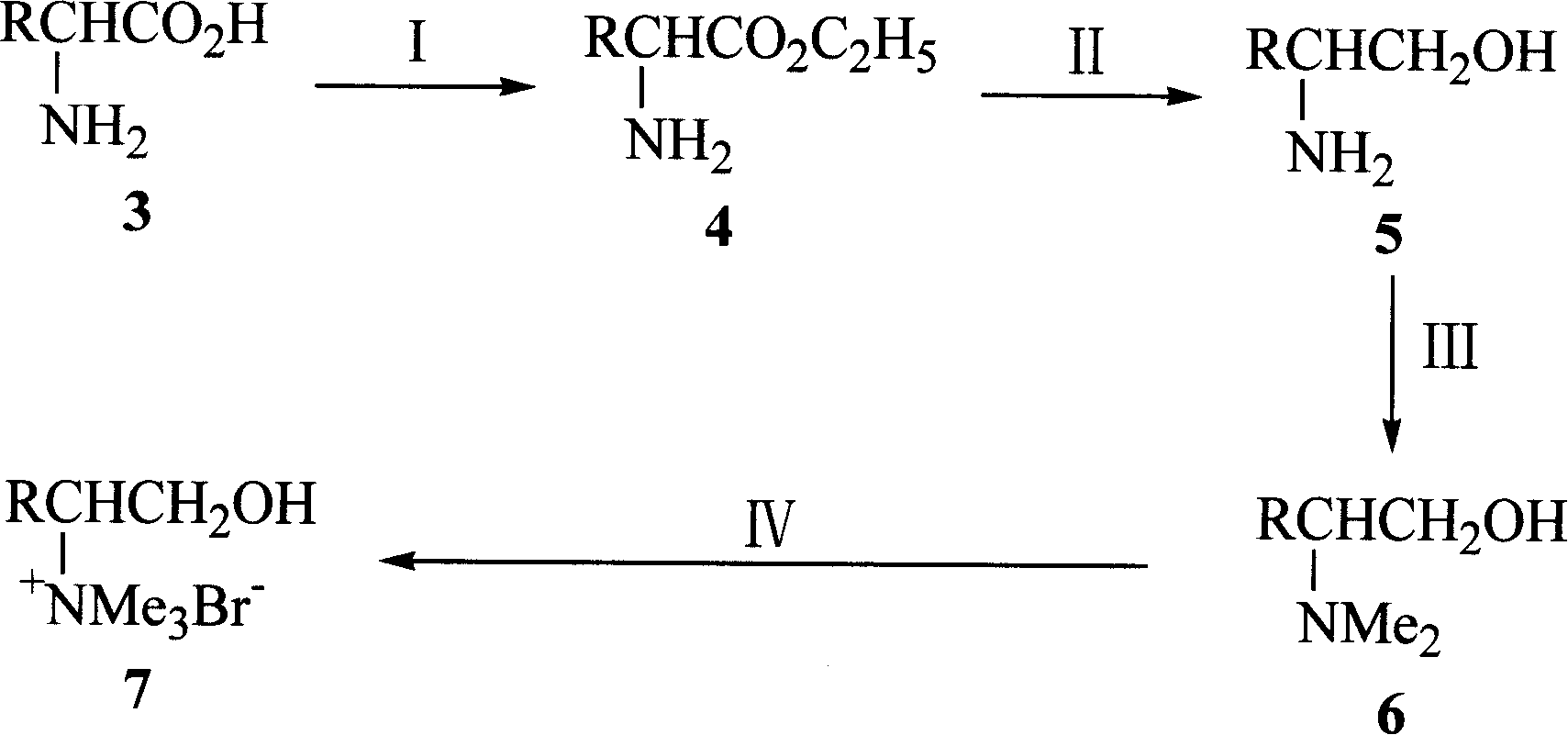
The present invention relates to one chiral amino acid derivative. L-amino acid as initial material is processed through four steps of esterification, carboxyl reduction, amino methylation and forming salt with bromomethane to synthesize the R-configuration chiral amino acid derivative. The derivative is used as novel chiral inclusion resolving agent and may be used in the resolving inclusion of ketoprofen as one non-steroid antiphlogistic medicine.
Owner:WENZHOU UNIVERSITY
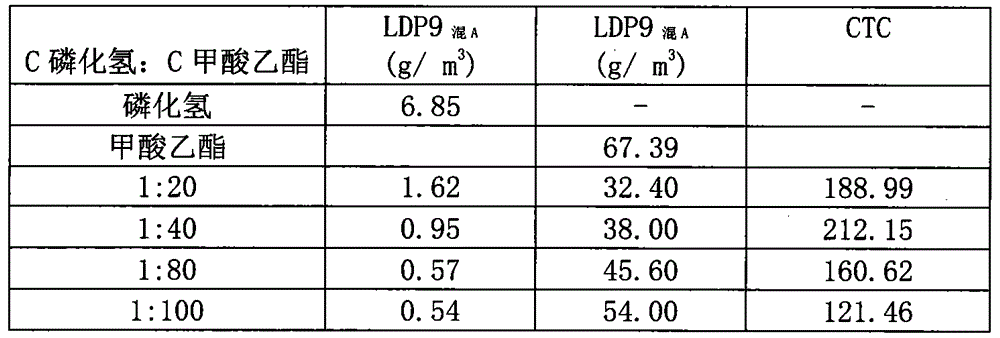
Phosphine and ethyl formate mixed synergist and application thereof
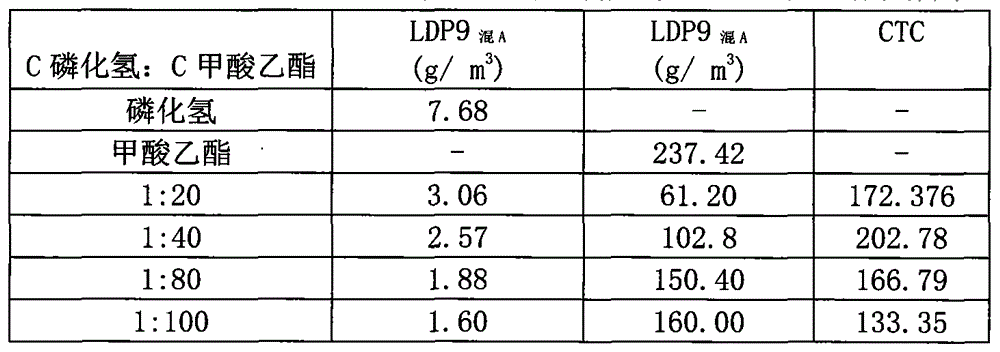
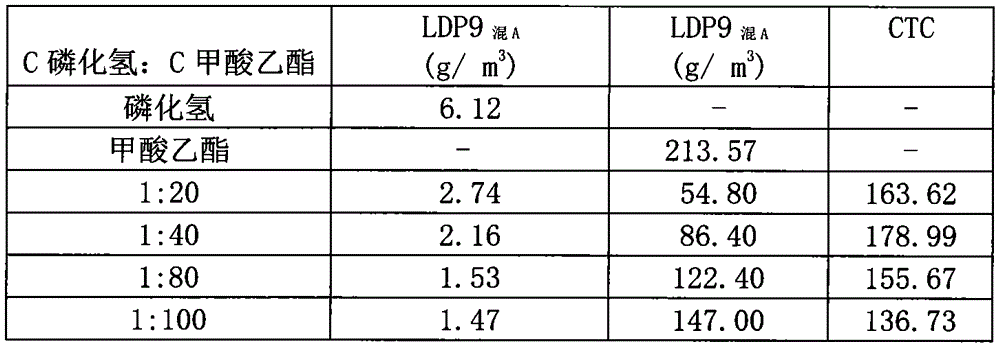
InactiveCN104886146AReduce usageGuaranteed tradeBiocideAnimal repellantsPest controlInsecticide resistance
The invention discloses a phosphine and ethyl formate mixed synergist for treating and killing pests carried by fresh agricultural products, and belongs to the technical field of application of fumigant mixing synergistic technology to pest control. According to the invention, the ratio of two fumigants and the fumigation temperature are mainly controlled to achieve an optimal synergistic effect, the usage amount of a single fumigant is effectively lowered, the defects that the phosphine treatment time is too long, the insecticide resistance is likely to generate, the quality of fresh agricultural products is prone to damage, the ethyl formate treating adsorption is overlarge, the dosage of ethyl formate is over high and the like are overcome, the operation is simple and convenient, and the application is easy. The phosphine and ethyl formate mixed synergist can be used for effectively preventing harm and propagation of fruits, vegetables, seedlings, flowers and the like carrying pests and keeping the quality of fresh agricultural products. A method with the phosphine and ethyl formate mixed synergist has the characteristics of high efficiency, economy, environment friendliness and the like, and is pest control technology capable of effectively replacing methyl bromide fumigation.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
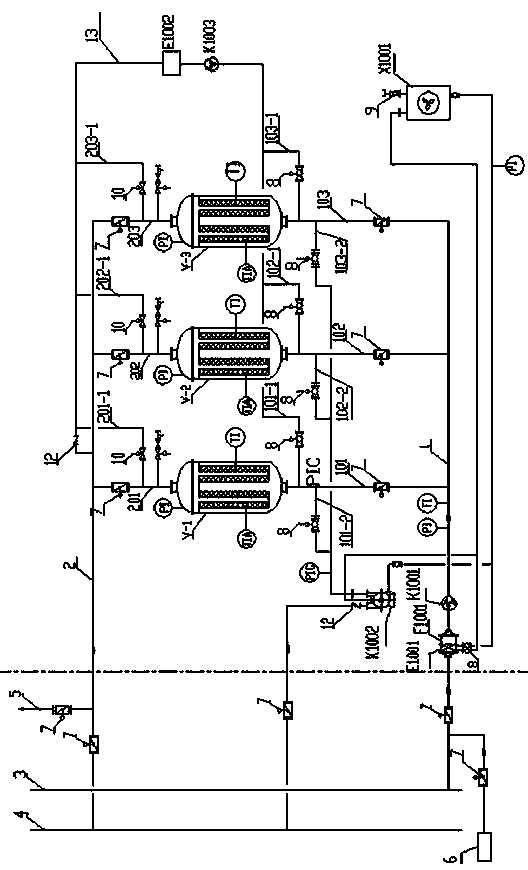
Fumigation agent methyl bromide activated carbon fiber adsorption recovery device and method thereof
ActiveCN104001406AEnable recyclingImprove the technical problems of recyclingDispersed particle filtrationActivated carbonPhysical chemistry
The invention relates to a fumigation agent methyl bromide activated carbon fiber adsorption recovery device. According to the fumigation agent methyl bromide activated carbon fiber adsorption recovery device, a plurality of ACF adsorption towers are connected to the same pipeline in parallel. The two ends of the pipeline are communicated with a fumigated base, so that a methyl bromide ACF circulation system is formed. The methyl bromide ACF circulation system is further communicated with a circulation heating system and a vacuum desorbing system. The method comprises the steps that a, methyl bromide gas in the fumigated base is introduced to the fumigation agent methyl bromide activated carbon fiber adsorption recovery device; b, a vacuum pump K1002 is started for vacuum desorbing; c, heat cycling is conduced; d, the ACF adsorption towers on which desorbing is conducted are prepared to conduct next adsorption; e, the desorbed methyl bromide gas is converted and sent to the base to be fumigated. Recycling and reusing of the fumigation agent methyl bromide are achieved after the fumigated base is fumigated, the fumigated base and a recycling and reusing device are organically combined, and recycling and reusing of the methyl bromide for fumigating of the fumigated base are achieved.
Owner:黄庆林 +1
Haloalkane activated molecular sieve with low boiling point and refining process thereof
InactiveCN102441366AReduce energy consumptionNo three wastes pollution problemOther chemical processesHalogenated hydrocarbon preparationNitrogen gasImpurity
The invention discloses a process for activating A-type or X-type molecular sieve under room temperature by using chloromethane (chloroethane). The activated molecular sieve is used for refining haloalkane with low boiling point such as chloromethane (chloroethane) and methyl bromide, and the refining process comprises the following steps: an exothermic reaction between the crystals in the molecular sieve and the chloromethane (chloroethane) is processed with a chemical equation of H2O+RCL->ROH+HCL+Q, in which the generated alcohol and hydrogen chloride are absorbed by alkali liquor and the unreacted chloromethane (chloroethane) are recycled after being condensed. After being fully purged by high-purity nitrogen, the activated molecular sieve is placed into a molecular sieve tower of the haloalkane refining system and then the haloalkane with low boiling point is refined according to the processes of adding the haloalkane liquid-phase absorbent, gasifying, alkali washing and acid removing, cooling and impurity removing, drying and dewatering, removing the molecular sieve and fully condensing and liquefying so as to refine the finished products with high purity.
Owner:陈锚
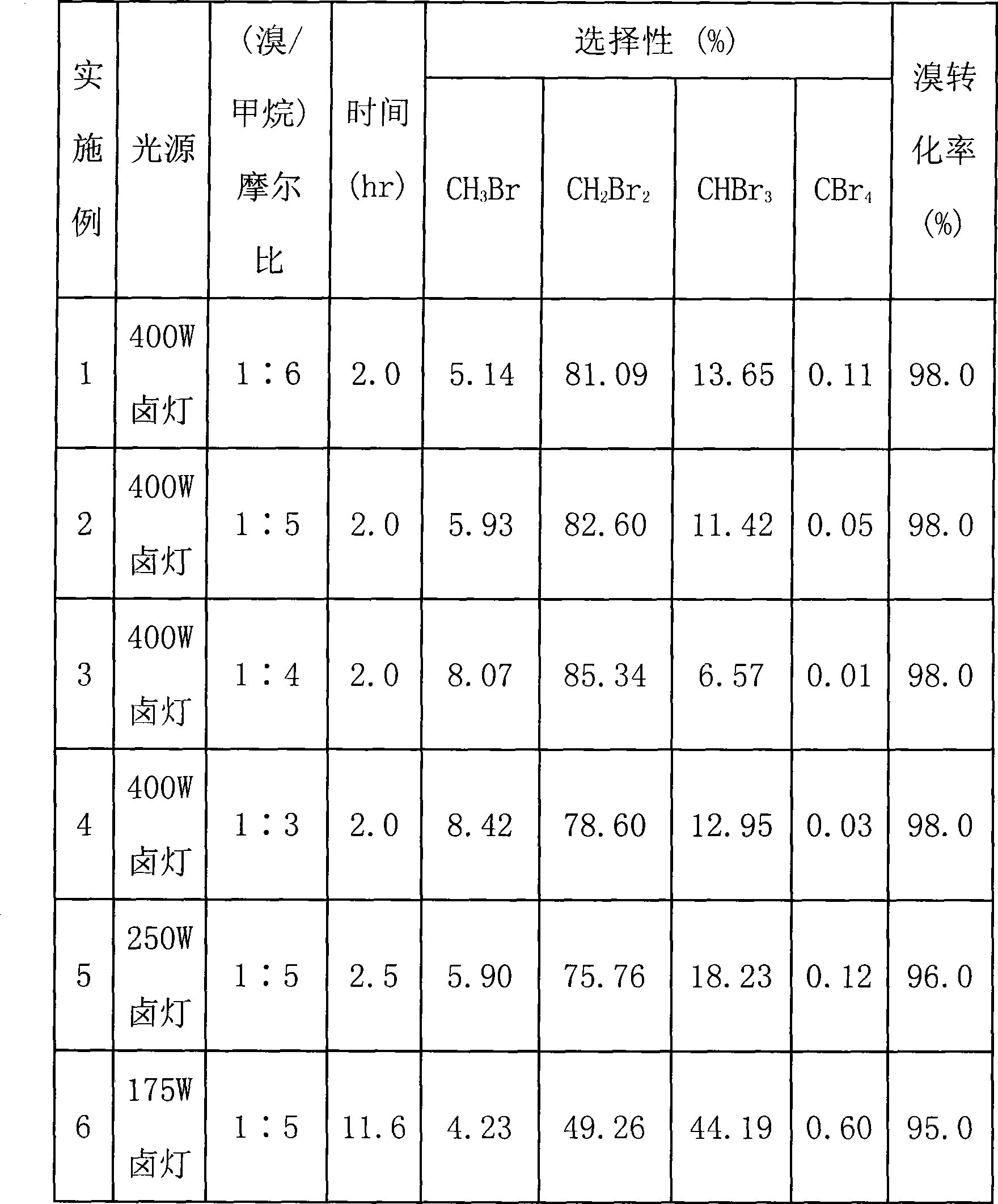
Process for synthesis of partially substituted bromomethane with photochemical reaction
InactiveCN101367706AThe principle of chemical reaction is simpleAdapt to industrial productionHalogenated hydrocarbon preparationChemical reactionBrownish red color
The invention discloses a technique of synthesizing low-substituted methyl bromide with photochemical reaction. The technique is provided with a photoreaction area and a circular condensing area. A reactor in the photoreaction area is vacuum before the reaction and can be illuminated by light and heated. The circular condensing area can be communicated with or is disconnected with the reactor, which is provided with a hydrogen bromide absorption tube and refrigerating circulation liquid. The circular condensing area is also connected with a product storage tank. When the reactor is vacuum, the reactor is filled with liquid bromine and methane. Light source is opened and the photoreaction begins. When the brownish red color of the liquid bromine in the reactor disappears, the reactor is heated at once to vaporize the reaction product completely. At the moment, the circular condensing area is communicated to exchange gas circularly for the product. The low-substituted methyl bromide is obtained in the product storage tank. The invention takes visible light as the light source. The chemical reaction principle is simple. The invention is applicable to commercial process.
Owner:EAST CHINA NORMAL UNIV
Production method of tetramethyl divinyl disiloxane
Owner:衢州瑞力杰化工有限公司
Trans-, cis-or cis-trans-1, 3-dichloropropene compound and rectification method thereof
PendingCN110845298APromote growthHigh rate of pest controlBiocideAgriculture tools and machinesChemical industryMeth-
The invention belongs to the technical field of chemical industry, and discloses a trans-1, 3-dichloropropene compound, a cis-1, 3-dichloropropene compound, a cis-trans-1, 3-dichloropropene compound and a rectification method thereof, the dichloropropene compound is composed of the trans-1, 3-dichloropropene and the cis-1, 3-dichloropropene or the cis-trans-1, 3-dichloropropene; the trans-1, 3-dichloropropene is mainly used for preparing chloramine, and the chloramine is used for preparing final product clethodim. The cis-trans mixed 1, 3-dichloropropene is used as a soil fumigant and a soil insecticide, radically treats underground nematodes and replaces methyl bromide in a methyl bromide project of the United Nations. The cis 1, 3-dichloropropene and a cis-trans mixed 1, 3-dichloropropene product are blended to serve as the cis-trans mixed 1, 3-dichloropropene to be still used for radically treating the underground nematodes. The dichloropropene compound can inhibit or directly killvarious nematodes and relieve nematode disease damage, the control effect reaches 98% or above, and the lasting period is 3 months or above.
Owner:湖南莱万特化工有限公司 +1
Synthesis method of 3-cyclic ether methyl trifluoro-potassium borate
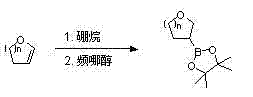
ActiveCN103896975ALow priceReduce manufacturing costGroup 3/13 element organic compoundsPtru catalystCyclic ether
The invention relates to 3-cyclic ether methyl trifluoro-potassium borate, in particular relates to a synthesis method of 3-cyclic ether methyl trifluoro-potassium borate, mainly solving the technical problems of the existing preparation method that the catalyst is expensive, the reaction condition is severe, the synthesis process is long and the like. According to the technical scheme, the synthesis method comprises the following steps: performing an addition reaction on 2-cyclic ether olefin taken as a raw material and borane; quenching with pinacol so as to obtain 3-cyclic ether-based pinacol boric acid ester; removing protons from the 3-cyclic ether-based pinacol acid ester by using a strong alkali and reacting with chlorobromomethane so as to obtain 3-cyclic ether methyl pinacol boric acid ester; and finally, performing a reaction on the 3-cyclic ether methyl pinacol boric acid ester and potassium bifluoride so as to obtain the 3-cyclic ether methyl trifluoro-potassium borate. The 3-cyclic ether methyl trifluoro-potassium borate is an important medicinal compound structure-modified micromolecule in the research field of a novel medicine.
Owner:SUNDIA MEDITECH COMPANY LTD
Synthetic method of 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane
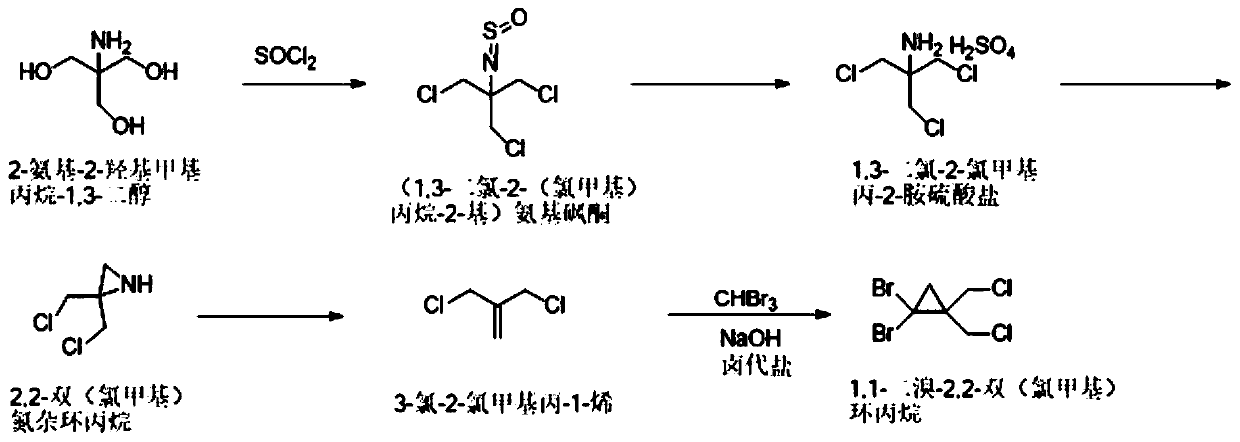
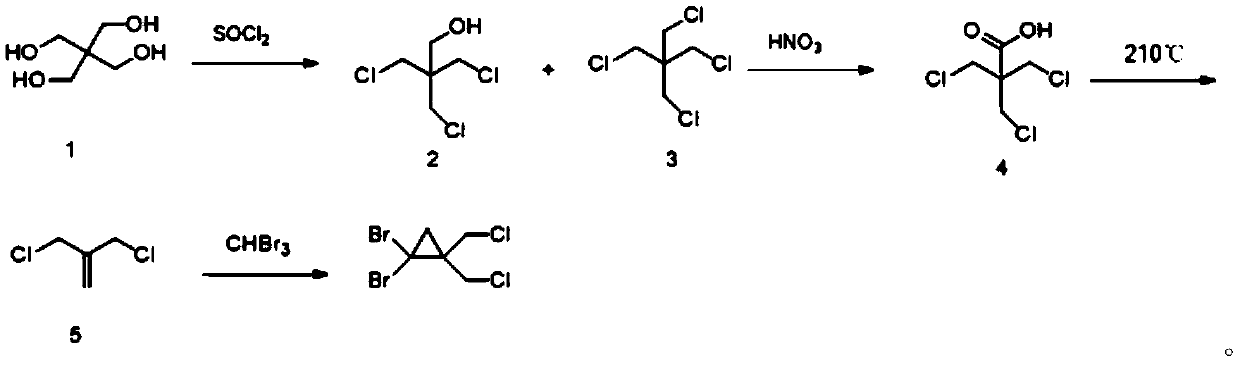
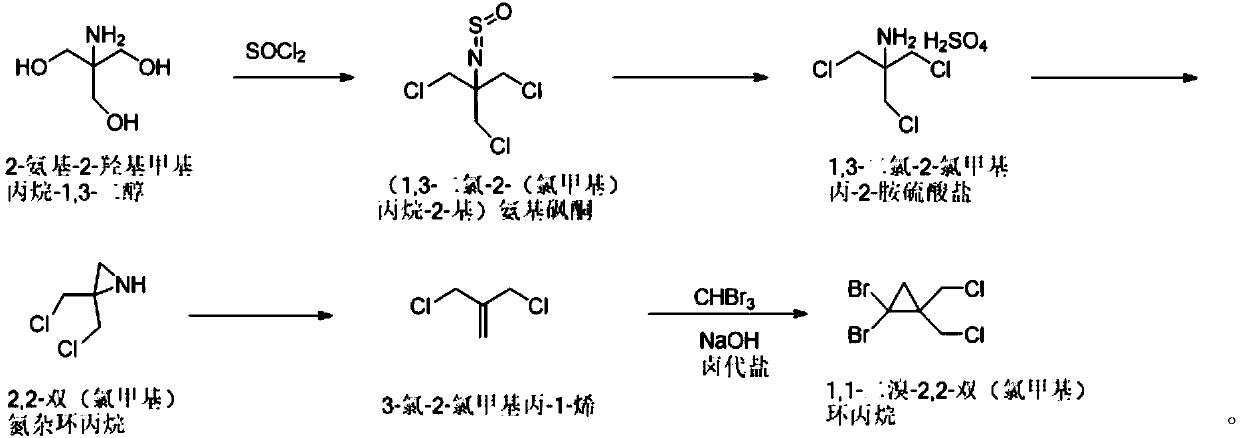
ActiveCN110759840AReduce instant heat releaseGuaranteed reaction rateOrganic compound preparationAmino compound preparationAziridinePropylamine
The invention discloses a synthetic method of 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane. The method comprises the following steps that chlorine substitution reaction happens to 2-amino-2-hydroxylmethylpropane-1,3-diol to obtain (1,3-dichloro-2-(chloromethyl)propane-2-group)amino-sulfone-ketone; the (1,3-dichloro-2-(chloromethyl)propane-2-group)amino-sulfone-ketone is hydrolyzed through a concentrated sulfuric acid aqueous solution to obtain 1,3-dichloro-chloromethylpropyl-2-amine-sulfate; the 1,3-dichloro-chloromethyl-propyl-2-amine-sulfate is subjected to salt dissolution on the alkaline condition, and is subjected to cyclization to form 2,2-dual(chloromethyl)aziridine; reaction happens to 2,2-dual(chloromethyl)aziridine and sodium nitrite to form 3-chloro-2-chloromethyl-propyl-1-alkene; and the 3-chloro-2-chloromethyl-propyl-1-alkene reacts with bromoform on the alkaline condition to generate 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane. The synthetic method of 1,1-dibromo-2,2-dual(chloromethyl)-cyclopropane is simple and can be used for enlarged production.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Waterproof paint not liable to peel off
InactiveCN103614056AReduce pollutionWon't peel offAnti-corrosive paintsPolyether coatingsKaolin clayEthyl group
The invention discloses waterproof paint not liable to peel off. The paint is prepared from following raw materials by weight: 2.5-3.8 parts of dibromomethane, 3.5-3.7 parts of tetrahydrofuran, 3.5-4.7 parts of nonylphenol polyoxyethylene ether, 3-7 parts of kaolin, 1.2-1.7 parts of phenylethyl naphthol polyoxyethylene ether, 2.5-5 parts of octadecanol polyoxyethylene ether, 1.2-2.2 parts of N-methyl-alkylamido taurine salt and 1.8-3.6 parts of a sorbitol-fatty acid ester. Compared with waterproof paint at present, the paint provided by the invention has high water resistance, high water proofness and high tensile strength and is healthy and environmental friendly; environment pollution is reduced and wall peeling off caused by rain wash after longtime use is avoided; the paint provided by the invention can prevent being washed by acid rain; and the paint film wound not peel off after rain wash, thus reducing the probability that rain permeates into the wall and corrodes the wall to cause losses.
Owner:QINGDAO HAIBAN PLASTIC IND & TRADE
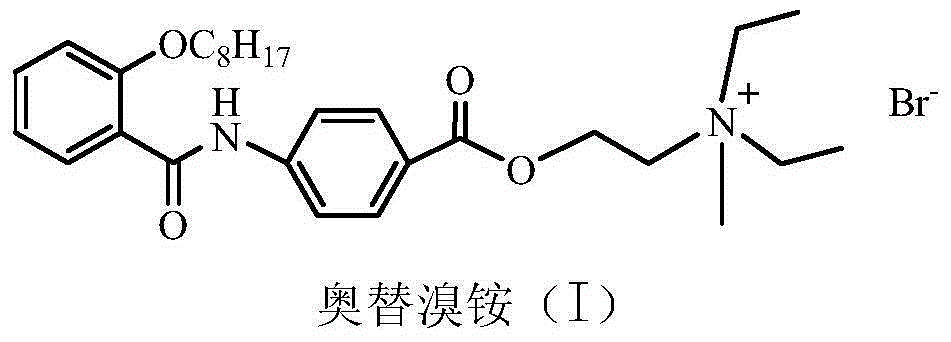
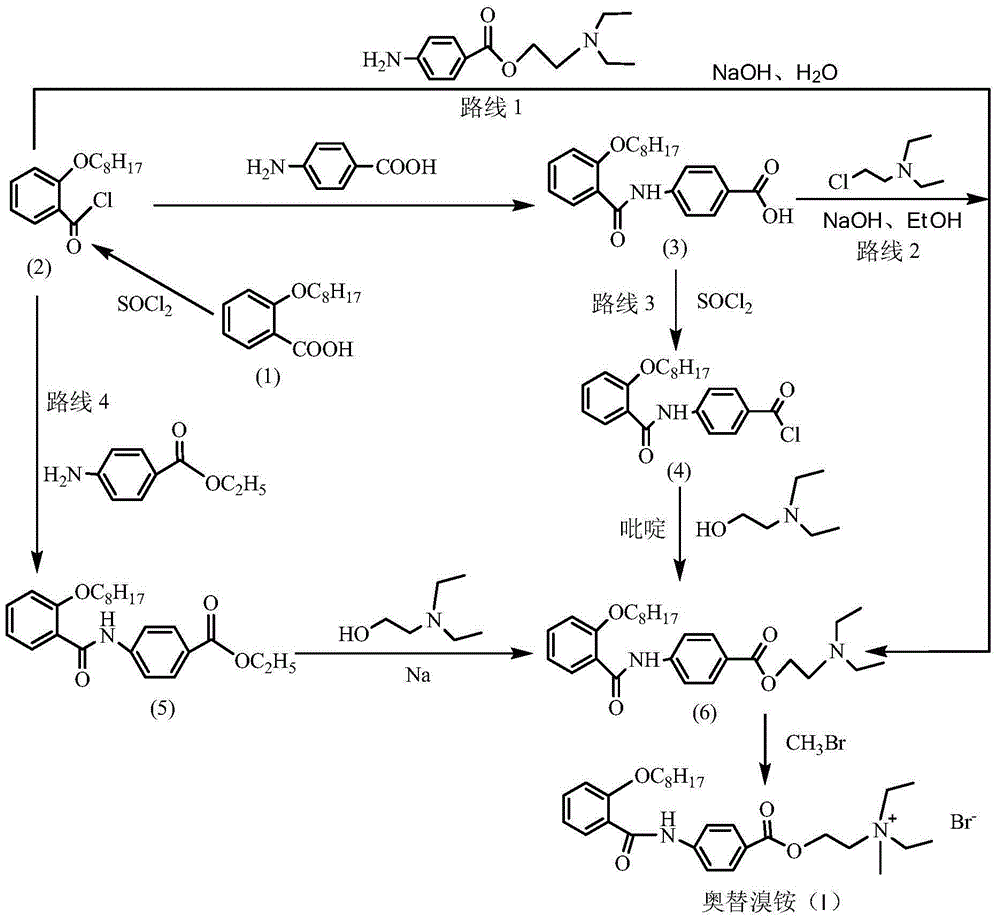
Preparation method of otilonium bromide
InactiveCN105037193AHigh purityHigh yieldOrganic compound preparationCarboxylic acid amides preparationBenzoic acidOtilonium bromide
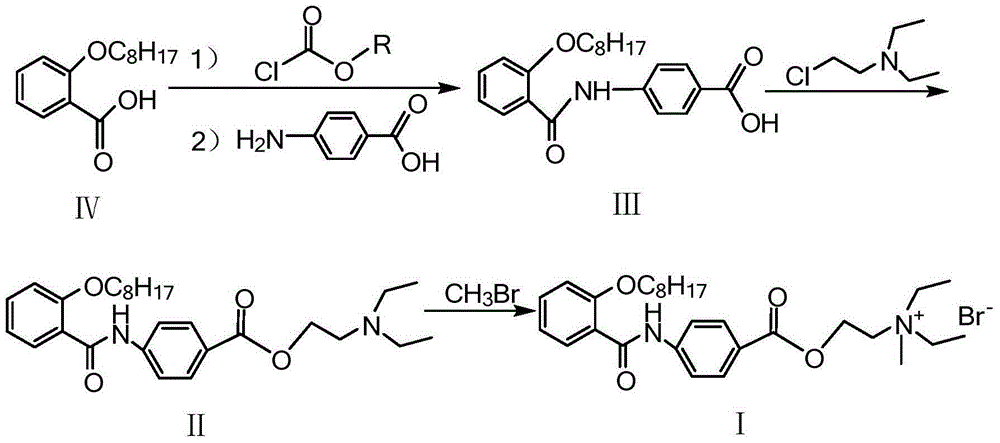
The invention provides a preparation method of otilonium bromide, belonging to the technical field of medicine production. The method comprises the following steps: reacting o-octanoxy benzoic acid serving as a raw material and chloroformate to obtain a product; then, carrying out condensation on the product and p-aminobenzoic acid to obtain 4-(2-octanoxybenzoylamino)benzoic acid; reacting 4-(2-octanoxybenzoylamino)benzoic acid and diethylaminoethyl chloride to obtain N,N-diethyl-2-[4-(2-octanoxybenzoylamino)benzoyloxy]ethylamine; and reacting N,N-diethyl-2-[4-(2-octanoxybenzoylamino)benzoyloxy]ethylamine and bromomethane to generate otilonium bromide. The preparation method has the advantages of simple process, low reaction byproduct content, high yield, simplicity and convenience in operation, economical efficiency, environmental friendliness and high industrial popularization value so as to be suitable for large-scale industrial production.
Owner:ZHEJIANG SANMEN HYGECON PHARMA CO LTD
Alkaline earth metal modified supported catalyst as well as preparation method and application thereof
ActiveCN106140231AHigh selectivityHigh activityPhysical/chemical process catalystsHydrocarbon from halogen organic compoundsPtru catalystAlkaline earth oxides
The invention provides an alkaline earth metal modified supported catalyst as well as a preparation method and application thereof. The alkaline-earth metal modified supported catalyst comprises zinc oxide, zinc halide, an alkaline earth metal oxide and an alumina supporter; in percentage by weight of the catalyst, zinc oxide accounts for 0.5-20%, zinc halide accounts for10-50%, the alkaline earth metal oxide accounts for 0.1-20%, and the balance is the alumina supporter. The preparation method of the catalyst comprises the following steps: (1) loading a zinc element precursor and an alkaline earth metal precursor on the alumina supporter with a co-impregnation method, and performing drying and roasting to obtain a catalyst precursor; (2) performing halogenation treatment on the catalyst precursor obtained in the step (1), thereby preparing the alkaline-earth metal modified supported catalyst . When the alkaline-earth metal modified supported catalyst is used in reaction of preparing isobutene from methyl bromide, the selectivity of isobutene can be remarkably improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com