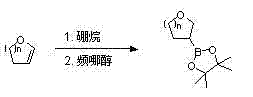
Synthesis method of 3-cyclic ether methyl trifluoro-potassium borate
A technology of potassium methyl trifluoroborate and its synthesis method, which is applied in the fields of compounds containing group 3/13 elements of the periodic table, chemical instruments and methods, organic chemistry, etc., and can solve environmental heavy metal pollution, increased production costs, and difficult raw materials To achieve cheap price, simple post-processing, good maneuverability and repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) 3-tetrahydrofuryl pinacol borate
[0030] In a dry 1000mL three-necked flask, add 25 g of 2,3-dihydrofuran and 120 mL of anhydrous tetrahydrofuran, cool the reaction solution to 0°C, and slowly add 360 mL of 1M borane / tetrahydrofuran solution dropwise into the reaction solution. 44 g of pinacol was dissolved in 100 mL of tetrahydrofuran, and slowly added dropwise to the reaction solution. The reaction solution was stirred overnight at room temperature. Water was slowly added dropwise to the reaction solution, extracted with ethyl acetate, the organic phase was concentrated and then purified by flash column chromatography to obtain 29 g of 3-tetrahydrofuryl pinacol borate with a yield of 41%. 1 H-NMR (300 MHz, CDCl 3 ): δ3.93-3.96 (m, 1H), 3.75-3.81 (m, 1H), 3.56-3.71 (m, 2H), 1.97-2.02 (m, 1H), 1.76-1.87 (m, 1H), 1.54 -1.60 (m, 1H), 1.25 (s, 12H).
[0031] ) 3-tetrahydrofuranmethyl pinacol borate
[0032] In a dry 500mL three-necked flask, add 29g of 3-tetra...
Embodiment 2
[0036] 1) 3-tetrahydropyranyl pinacol borate
[0037] In a dry 1000mL three-necked flask, add 25 g of 3,4-dihydropyran and 120 mL of anhydrous tetrahydrofuran, cool the reaction solution to 0°C, and slowly add 300 mL of 1M borane / tetrahydrofuran solution dropwise into the reaction solution . 35 g of pinacol was dissolved in 100 mL of tetrahydrofuran, and slowly added dropwise to the reaction solution. The reaction solution was stirred overnight at room temperature. Water was slowly added dropwise to the reaction solution, extracted with ethyl acetate, the organic phase was concentrated and then purified by flash column chromatography to obtain 28 g of 3-tetrahydropyranyl pinacol borate with a yield of 45%. 1 H-NMR (300 MHz, CDCl 3 ): δ3.78-3.89 (m, 2H), 3.40-3.51 (m, 2H), 1.79-1.82 (m, 1H), 1.48-1.57 (m, 3H), 1.29-1.32 (m, 1H), 1.23 (s, 12H).
[0038] ) 3-Tetrahydropyranylmethyl pinacol borate
[0039] Preparation of lithium diisopropylamide (LDA): In a dry 500 mL thre...
Embodiment 3
[0044] 1) 2-(4-chlorophenyl)-4-(3-tetrahydrofurylmethyl)thieno[2,3-d]pyrazine-7-carboxamide
[0045] In a dry 250 mL three-necked flask, 4-chloro-2-(4-c chlorophenyl)-thieno[2,3-d]pyrazine-7-carboxamide (10.0 g) was added under nitrogen protection, Anhydrous tetrahydrofuran (100 mL) and 1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (1.13g), then potassium 3-tetrahydrofuranmethyl trifluoroborate (6.53g), The reaction mixture was stirred overnight at 60 °C. After cooling to room temperature, saturated aqueous ammonium chloride solution was added, the layers were separated, and the aqueous layer was extracted with ethyl acetate. After combining the organic layers, they were washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to remove the solvent to obtain a crude product. The crude product was purified by column chromatography on silica gel (petroleum ether / ethyl acetate volume ratio=5 / 1) to obtain 7.04 g of 2-(4-chlorophenyl)-4-(3-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com