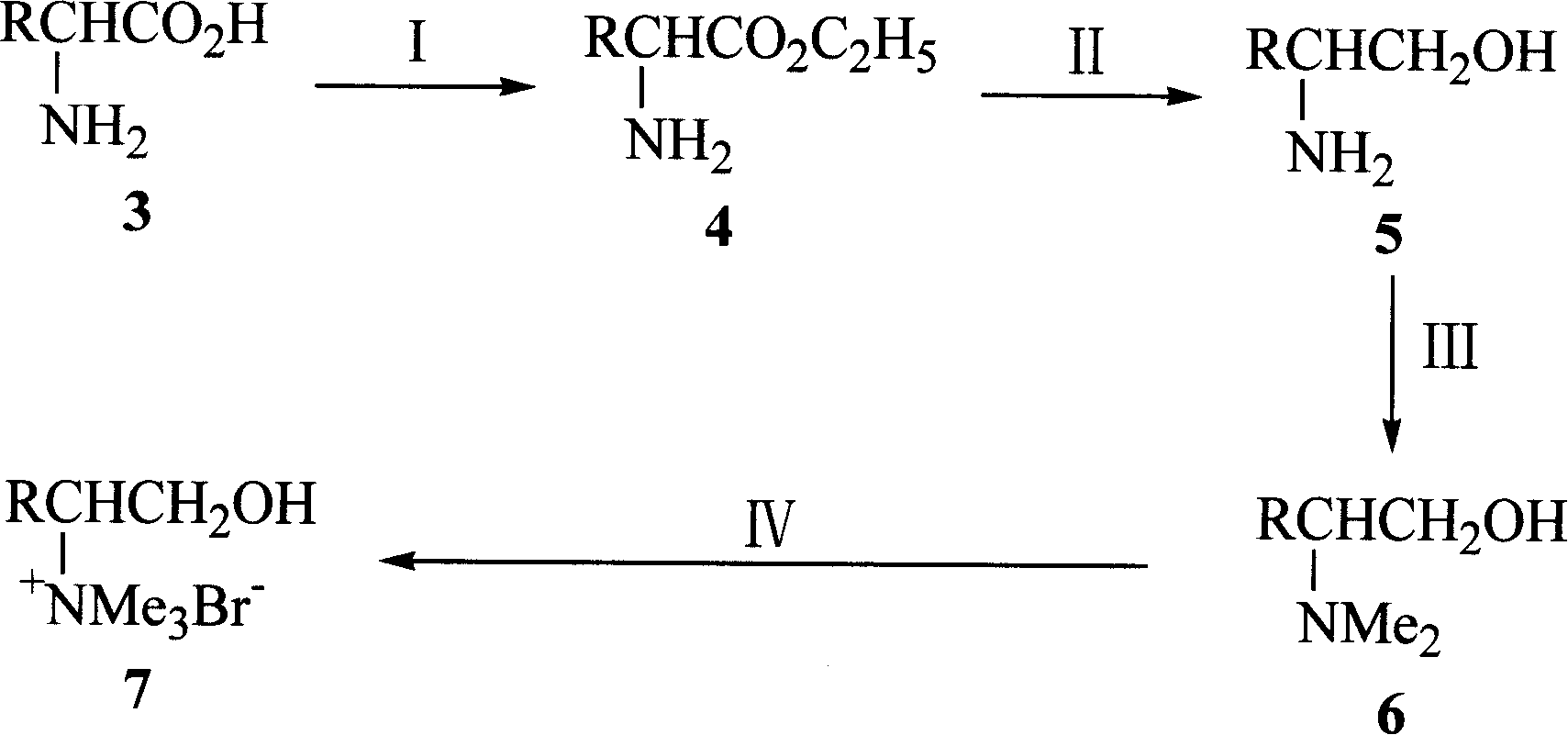
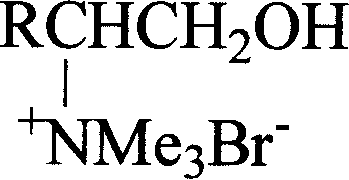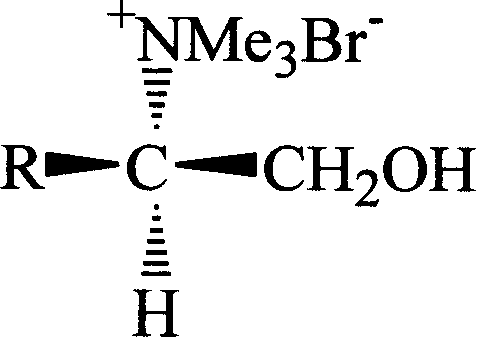Novel chiral amino acid derivative and its synthetic method and use
A technology of chiral amino acid and synthesis method, which is applied in the field of chiral amino acid derivatives, can solve the problems of high price and unsuitability for large-scale splitting, and achieve the effect of simple and effective method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0031] Embodiment 1-2; Synthesis of L-phenylalaninol by L-phenylalanine ethyl ester
[0032] Method (1): 1000mL three-neck alkaloid is equipped with magnetic stirring, reflux condenser, constant pressure dropping funnel, and 166mmol NaBH is added to the flask 4 , 55mmol L-phenylalanine and 220ml anhydrous THF, the reaction flask was cooled to 0°C in an ice bath, and 60mmol TiCl was added within 30min 4 Slowly drop into the reaction mixture through the dropping funnel, hydrogen gas is generated, react overnight at room temperature, then reflux the reaction mixture for 3 hours until no gas is released, cool to room temperature, add 200ml of diethyl ether to dilute, and then add an appropriate amount of Stop the reaction with water, add NaOH (20%) 150mL and stir for 30min, filter, separate the organic layer, wash the filter cake with diethyl ether (3×20mL), combine the organic layers, anhydrous NaOH 2 SO 4 After drying, the solvent was evaporated under reduced pressure, and the...
Embodiment 1-4
[0035] Embodiment 1-4; By N, N-dimethyl-L-phenylalaninol synthetic trimethyl (1-hydroxyl-3-phenylpropan-2-yl) ammonium bromide
[0036] Add 10mmol of N,N-dimethyl-L-phenylalaninol, 15mmol of methyl bromide and 100mL of acetonitrile into a 250mL three-neck flask equipped with a stirring and reflux device, and stir for 6h in an ice-water bath. Atmospheric distillation recovers acetonitrile and excess methyl bromide. Remove the remaining small amount of acetonitrile and methyl bromide under reduced pressure at 100°C to obtain a light yellow solid, then add 50 mL of absolute ethanol to reflux for 0.5 h, and cool to crystallize. Filter and remove the remaining anhydrous ethanol from the product under reduced pressure at 50°C to obtain the product. The product is white crystal, and the yield is 95%. m.p.196~198℃; [α] D 20 =+4.40(c0.5, CH 3 OH); 1 H NMR (300MHz, D 2 O)δ: 2.86(dd, J=8.5, 13.4Hz, 1H, 3-H), 3.02(dd, J=5.3, 13.4Hz, 1H, 3-H), 3.20(s, 9H, N(CH 3 ) 3 ), 3.25~3.33(m...
Embodiment 2
[0037] Embodiment 2; Synthetic trimethyl (1-hydroxy propan-2-base) ammonium bromide by L-alanine synthesis
[0038] Trimethyl(1-hydroxypropan-2-yl)ammonium bromide can be prepared from L-alanine in the same manner as above. Yield 72%. m.p.127~129℃; [α] D 20 =-4.6(c0.5, CH 3 OH); 1 H NMR (300MHz, D 2 O)δ: 1.36~1.44(m, 3H, 3-H), 3.07(s, 9H, N(CH 3 ) 3 ), 3.82(dd, J=7.4, -10.4Hz, 1H, 1-H), 4.00(dd, J=4.0, -10.4Hz, 1H, 1-H), 4.02~4.14(m, 1H, 2- H),; IR (KBr) υ: 3292, 2966, 2920, 2893, 1668, 1596, 1458, 1378, 1311, 1263, 1218, 1147, 1066, 989, 984, 834, 798, 624, 619cm -1 . Anal.calcd for C 6 h 16 ONBr: C 36.38, H 8.14, N 7.07; found C 36.35, H 8.12, N 7.10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com