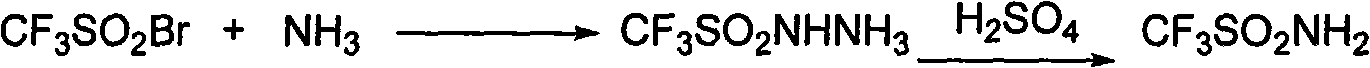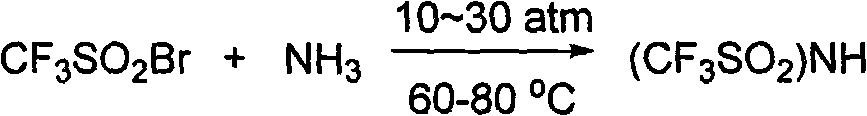Method for preparing sulfimide compound
A technology of sulfonimide salt compound and sulfonamide, applied in the preparation of sulfonamide, organic chemistry and other directions, can solve the problems of high price of trifluoromethanesulfonamide, difficulty in large-scale production, unsuitable for mass production, etc. Low cost, ease of operation, improved purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025]
[0026] CF 3 SO 2 NH 2 Synthesis:
[0027] After taking 1000mL of liquid ammonia, add 250mL of CF dropwise with a constant pressure dropping funnel 3 SO 2Br, a yellow substance was formed, the dropwise addition was completed in 1h, and the reaction was stopped after 3h to obtain a light yellow solid, and then the excess ammonia was allowed to volatilize naturally at room temperature. Prepare 50% sulfuric acid solution and adjust the pH=2 of the system. Then add 500mL of refined acetonitrile, shake to dissolve, then filter with suction, and wash twice with 150mL of acetonitrile. Acetonitrile was distilled off under reduced pressure to obtain a slightly yellow waxy solid.
[0028] CF 3 SO 2 NH 2 Refined:
[0029] CF will be produced 3 SO 2 NH 2 Recrystallize with refined toluene, use a reflux device, heat until it is completely dissolved when boiling, and precipitate crystals after cooling, obtain the product by suction filtration, dry and sto...
Embodiment 2
[0033] (CF 3 SO 2 ) 2 Synthesis of NLi:
[0034]
[0035] Take the refined CF 3 SO 2 NH 2 (7.5g, 50mmol) was added to a 500mL three-necked flask, then 200mL tributylamine was added to dissolve, heated to 35°C, CF 3 SO 2 NH 2 All dissolved. Use a pipette to measure 8 mL of CF 3 SO 2 Add Br to the dropping funnel, slowly add CF dropwise 3 SO 2 Br, the temperature was kept at 30°C, and the dropwise addition was completed in 1 hour, and the color of the solution gradually changed from reddish brown to dark red. Keep the reaction at 70°C for 6h, then distill off the solvent tributylamine under reduced pressure, and dissolve the residue in CH 2 Cl 2 , washed 3 times with water, then washed with anhydrous MgSO 4 The organic phase in the lower layer was dried, filtered with suction and then concentrated by vacuum distillation with a water pump to obtain a dark red oily substance. in N 2 Dissolve the obtained dark red oil with 250mL of methanol under protection, add...
Embodiment 3
[0038] Take the refined CF 3 SO 2 NH 2 (7.5g, 50mmol) was added to a 500mL three-necked flask, then 250mL triethylamine was added, heated to 50°C, CF 3 SO 2 NH 2 All dissolved. Measure 12mL of CF 3 SO 2 Add Br to the dropping funnel, slowly add CF dropwise 3 SO 2 Br, the temperature was kept at 50°C, and the dropwise addition was completed in 60 minutes, and the color of the solution gradually changed from reddish brown to dark red. Then the temperature is raised to 70°C, in order to make the CF 3 SO 2 NH 2 Fully reacted, add 1mL CF after 4h 3 SO 2 Br, and then reacted for 4h. Then evaporate the solvent on the rotary evaporator, the temperature of the water bath is 50 ° C, and the dark red oil remains, and the residue is dissolved in 200 mL CH 2 Cl 2 , washed 3 times with water, separated the lower CH 2 Cl 2 , with anhydrous MgSO 4 After drying and suction filtration, the product was distilled and concentrated under reduced pressure with a water pump to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com