Process for synthesis of partially substituted bromomethane with photochemical reaction
A photochemical reaction and low substitution technology, applied in the fields of organic chemistry, chemical instruments and methods, halogenated hydrocarbon preparation, etc., can solve the problems of low degree of green process, high cost of raw materials, poor selectivity, etc. The effect of low equipment cost and convenient source
Inactive Publication Date: 2009-02-18
EAST CHINA NORMAL UNIV
View PDF0 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
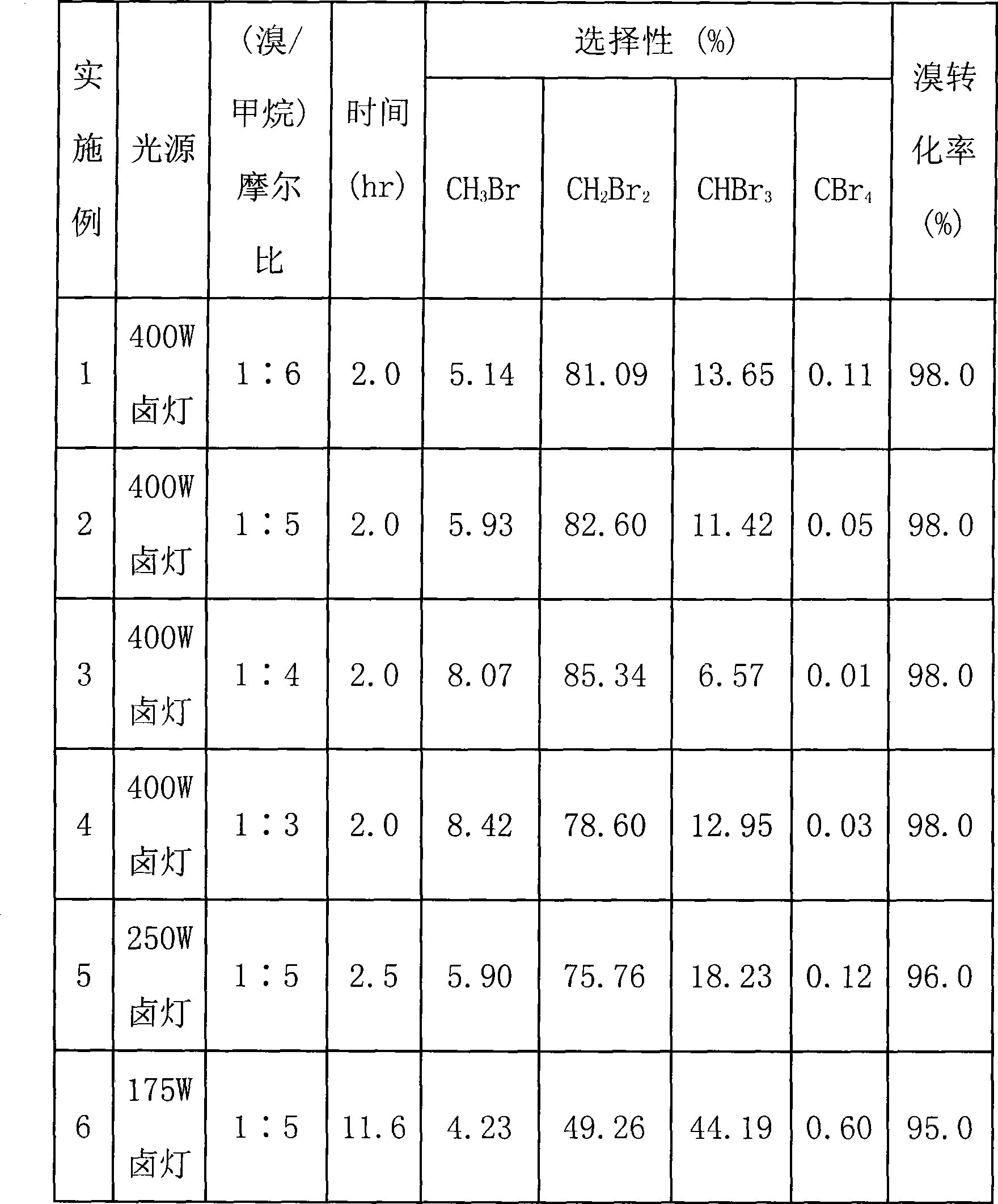
Embodiment 1-6
[0042]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
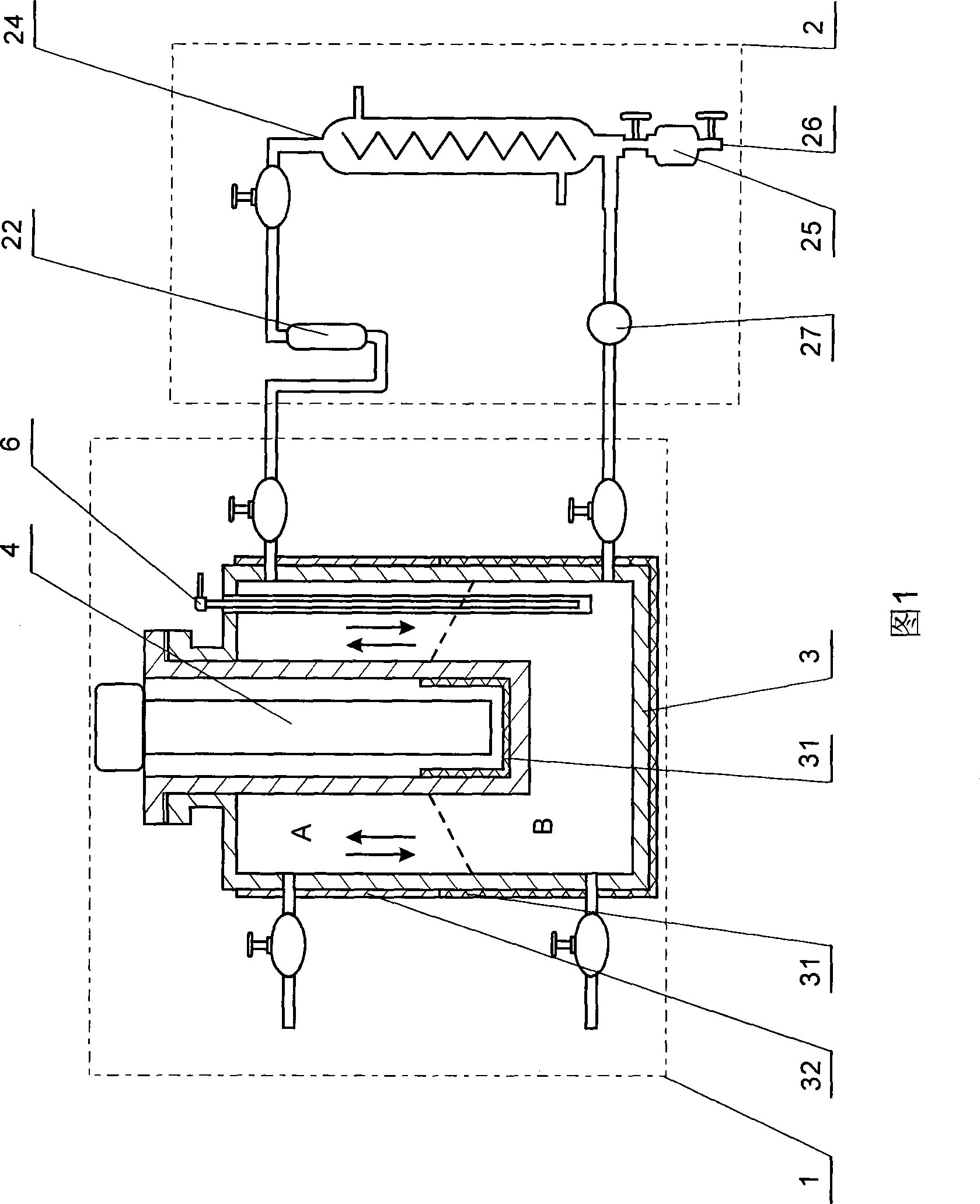
The invention discloses a technique of synthesizing low-substituted methyl bromide with photochemical reaction. The technique is provided with a photoreaction area and a circular condensing area. A reactor in the photoreaction area is vacuum before the reaction and can be illuminated by light and heated. The circular condensing area can be communicated with or is disconnected with the reactor, which is provided with a hydrogen bromide absorption tube and refrigerating circulation liquid. The circular condensing area is also connected with a product storage tank. When the reactor is vacuum, the reactor is filled with liquid bromine and methane. Light source is opened and the photoreaction begins. When the brownish red color of the liquid bromine in the reactor disappears, the reactor is heated at once to vaporize the reaction product completely. At the moment, the circular condensing area is communicated to exchange gas circularly for the product. The low-substituted methyl bromide is obtained in the product storage tank. The invention takes visible light as the light source. The chemical reaction principle is simple. The invention is applicable to commercial process.
Description
technical field [0001] The invention relates to the technical field of organic photochemical synthesis, in particular to a process for synthesizing low-substituted methyl bromide by photochemical reaction. Background technique [0002] Methane is the main component of coalbed methane and natural gas. With the increasing depletion of oil resources, abundant natural gas resources will become one of the most promising alternative energy sources and chemical raw materials in the future. [0003] The direct conversion of stable methane into high value-added chemicals has always been the dream of the scientific community at home and abroad, and it is also one of the most challenging topics in the field of heterogeneous catalysis. [0004] At present, there are different ways and methods to study methane (activation) conversion at home and abroad. From the current research situation, although some valuable results have been obtained, there is still a big gap with the goal of indus...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C19/075C07C17/10
Inventor 单永奎李疆孔爱国张恒强孟静
Owner EAST CHINA NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com