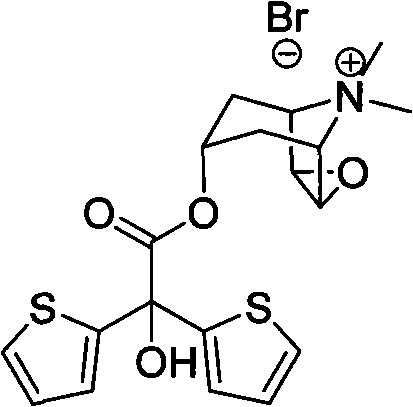
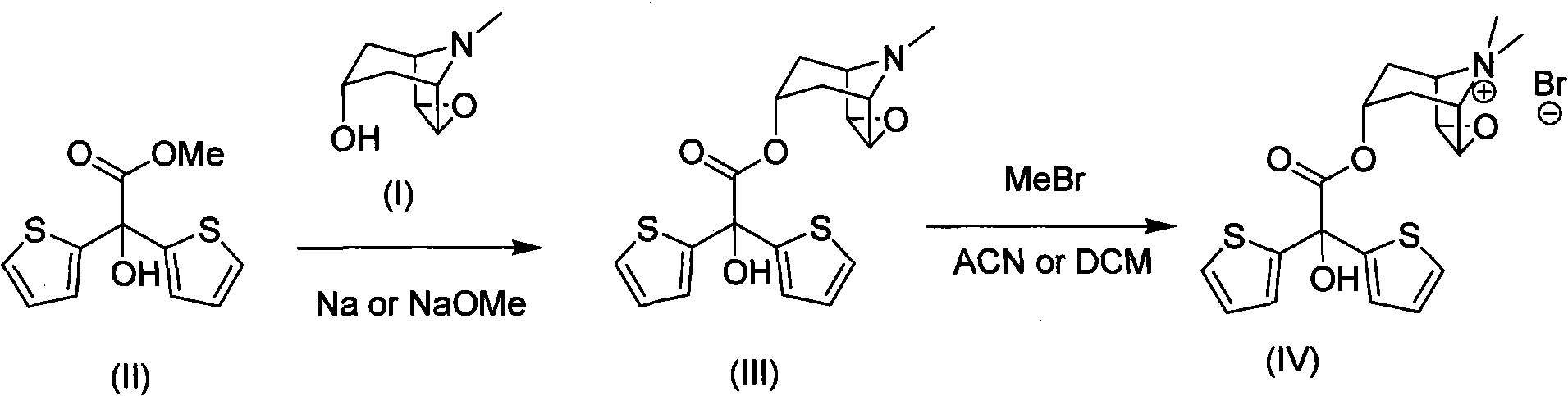
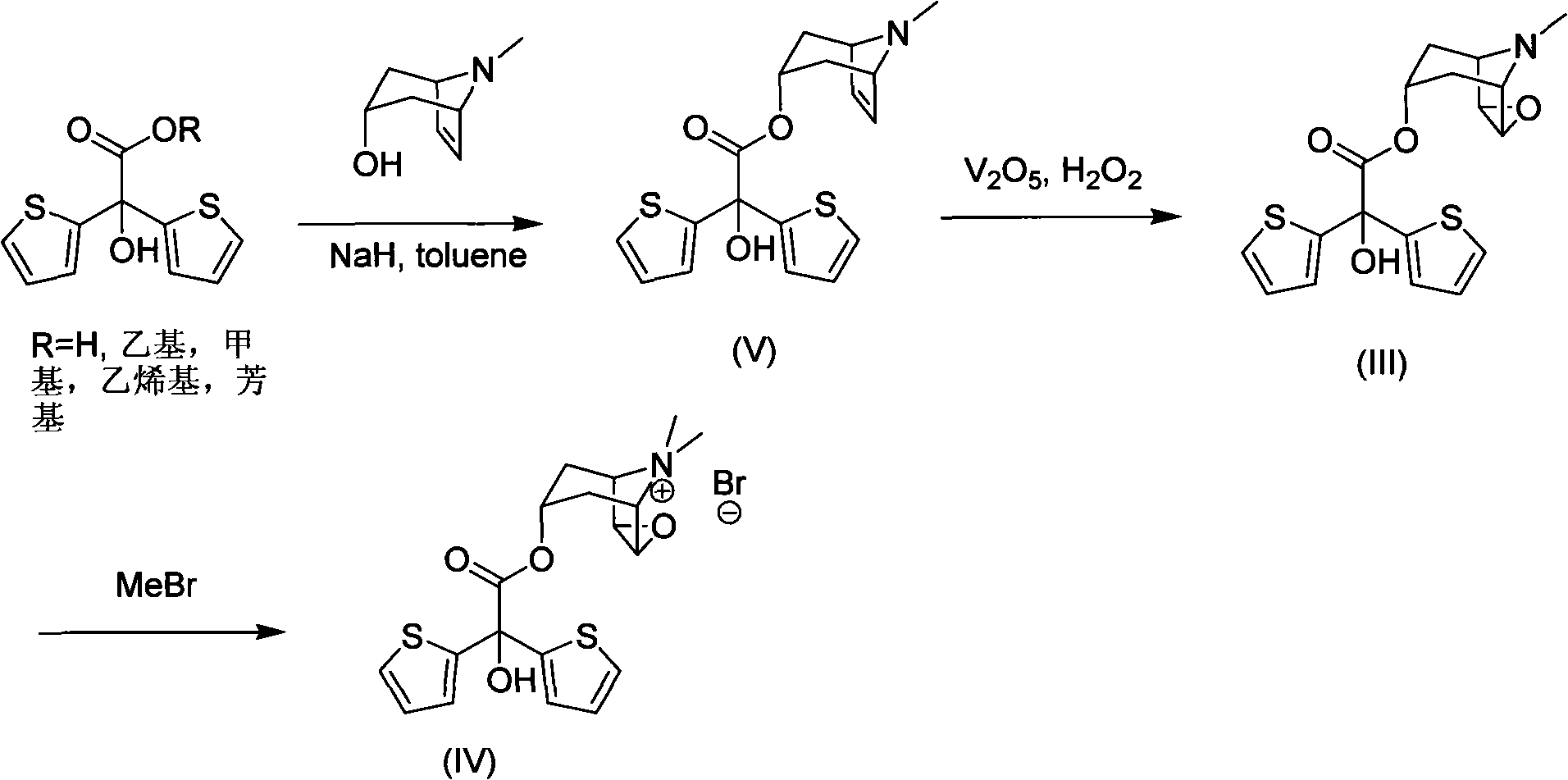
Method for preparing tiotropium bromide
A tiotropium bromide and thiophenol-based technology, applied in the field of preparation of tiotropium bromide, can solve problems such as harsh reaction conditions, unsuitability for large-scale production, etc., and achieve the effects of less environmental pollution, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of scopolamine bis(2-thiophene) glycolate.
[0037] In a dry three-necked flask, add 6.2g scopolamine (40mmol), 11.2g methyl bis(2-thiophenol) glycolate (44mmol), 0.5g anhydrous potassium carbonate (3.6mmol) and 50mL normal heptane. The three-necked bottle is equipped with a water separator and a condenser. Under nitrogen protection, the system was heated to reflux in an oil bath (oil bath temperature 134° C.) for 3 hours. Naturally cooled, 20 mL of ethyl acetate was added, potassium carbonate was filtered off, and washed with ethyl acetate. The filtrate was collected, evaporated to dryness, washed with acetonitrile, and the remaining solvent was removed in vacuo to obtain 12.2 g of scopolamine bis(2-thiophene)glycolate (yield 81%).
[0038] 1 H NMR (500MHz, CDCl 3 ), (ppm): 7.32-7.30(m, 2H), 7.13-7.12(m, 2H), 7.00-6.98(m, 2H), 5.14-5.12(m, 1H), 4.81(s, 1H), 3.07 -3.06(m, 2H), 3.00(s, 2H), 2.47(s, 3H), 2.17-2.11(m, 2H), 1.62-1.58(m, 2H).
Embodiment 2
[0039] Example 2: Preparation of scopolamine bis(2-thiophene) glycolate.
[0040] In a dry three-necked flask, add 3.1g scopolamine (20mmol), 5.6g bis(2-thienyl)glycolic acid methyl ester (22mmol), 0.5g anhydrous potassium carbonate (3.6mmol) and 50mL n-hexane alkyl. The three-necked bottle is equipped with a water separator and a condenser. Under nitrogen protection, the system was heated to reflux in an oil bath (oil bath temperature 134° C.) for 3 hours. Naturally cooled, 20 mL of ethyl acetate was added, potassium carbonate was filtered off, and washed with ethyl acetate. The filtrate was collected, evaporated to dryness, washed with acetonitrile, and the remaining solvent was removed by vacuum to obtain 4.9 g of scopolamine bis(2-thienyl)glycolate (65% yield).
[0041] 1 H NMR (500MHz, CDCl 3 ), (ppm): 7.32-7.30(m, 2H), 7.13-7.12(m, 2H), 7.00-6.98(m, 2H), 5.14-5.12(m, 1H), 4.81(s, 1H), 3.07 -3.06(m, 2H), 3.00(s, 2H), 2.47(s, 3H), 2.17-2.11(m, 2H), 1.62-1.58(m, 2H). ...
Embodiment 3
[0042] Example 3: Preparation of scopolamine bis(2-thiophene) glycolate.
[0043]In a dry three-necked flask, add 3.1g scopolamine (20mmol), 5.6g methyl bis(2-thiophene) glycolate (22mmol), 0.5g anhydrous potassium carbonate (3.6mmol) and 50mL normal heptane. The three-necked bottle is equipped with a water separator and a condenser. Under nitrogen protection, the system was heated to reflux in an oil bath (oil bath temperature 134° C.) for 3 hours. After natural cooling, 20 mL of methyl tetrahydrofuran was added, potassium carbonate was filtered off, and washed with ethyl acetate. The filtrate was collected, evaporated to dryness, washed with acetonitrile, and the remaining solvent was removed in vacuo to obtain 5.9 g of scopolamine bis(2-thienyl)glycolate (yield 78%).
[0044] 1 H NMR (500MHz, CDCl 3 ), (ppm): 7.32-7.30(m, 2H), 7.13-7.12(m, 2H), 7.00-6.98(m, 2H), 5.14-5.12(m, 1H), 4.81(s, 1H), 3.07 -3.06(m, 2H), 3.00(s, 2H), 2.47(s, 3H), 2.17-2.11(m, 2H), 1.62-1.58(m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com