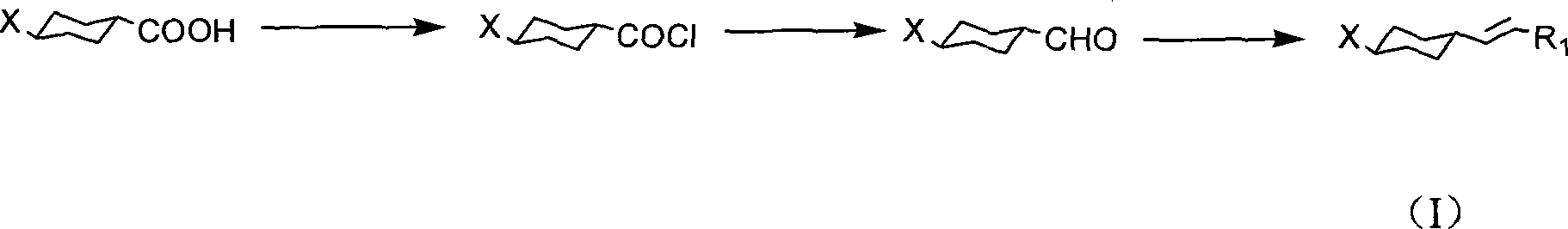
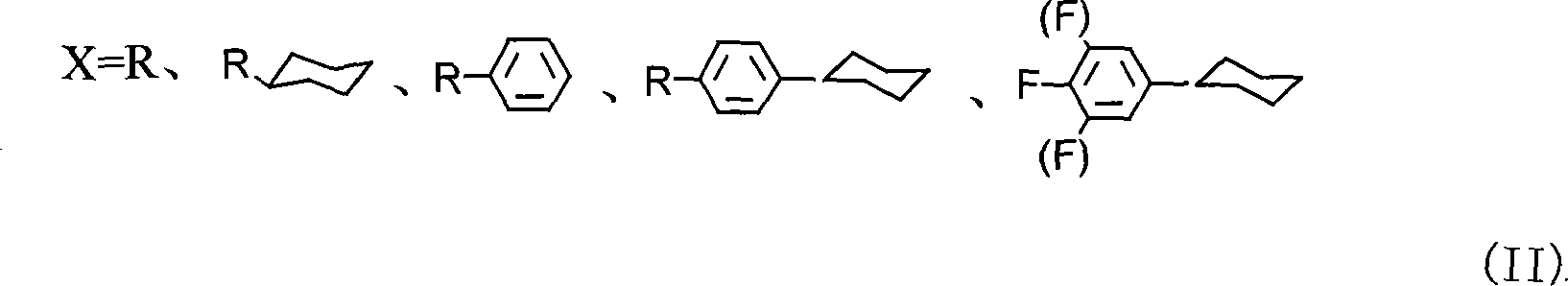
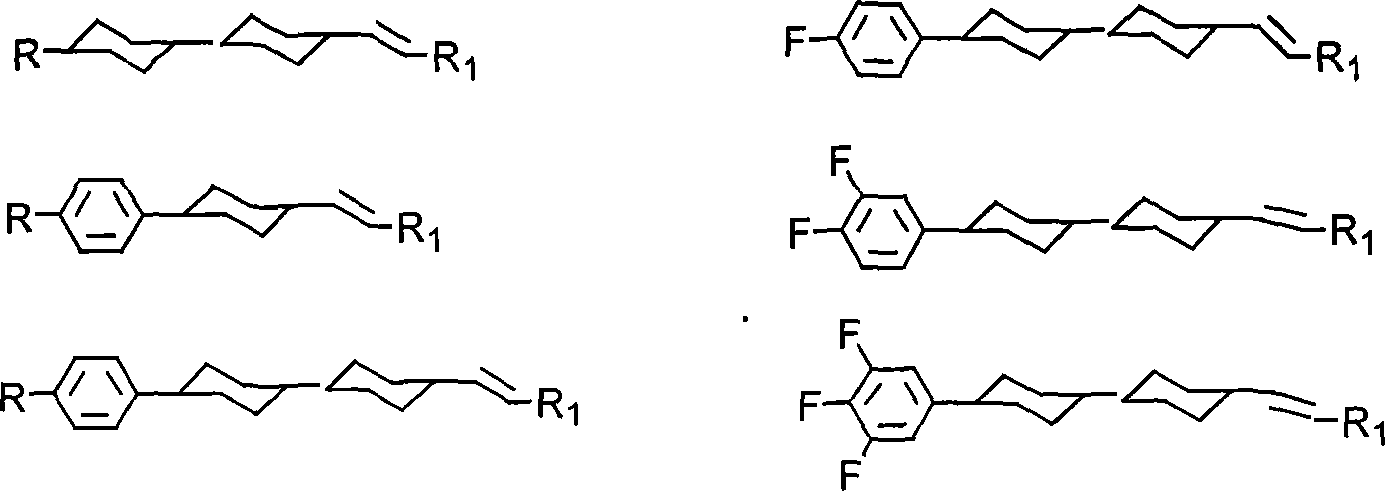
Method for producing cyclohexyl group olefin hydrocarbon liquid crystal material
A cyclohexyl olefin and cyclohexyl technology, applied in the direction of liquid crystal materials, chemical instruments and methods, hydrocarbons, etc., can solve the problems of easy structural conversion, pollution, serious pollution, etc., achieve small optical anisotropy and wide application Prospect, effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, preparation trans, trans-4-(4-propylcyclohexyl) cyclohexylethylene
[0046] 1. Preparation of trans, trans-4-(4-propylcyclohexyl) cyclohexylcarbonyl chloride
[0047] In the reflux condenser with drying tube, in a 250mL three-necked flask, add trans, trans-4-(4-propylcyclohexyl)cyclohexyl formic acid (0.1mol), thionyl chloride (0.15mol), benzene ( 100 mL), stirred and refluxed for 10 hours. The reaction mixture was rotary evaporated to remove the solvent, and the solvent and thionyl chloride were spin-dried as much as possible, and sealed for later use.
[0048] 2. Preparation of trans, trans-4-(4-propylcyclohexyl)cyclohexylcarbaldehyde
[0049] In a 500ml three-necked flask, add trans, trans-4-(4-propylcyclohexyl)cyclohexylcarbonyl chloride and 200mlTHF prepared in the previous step, under nitrogen protection, add 2.8g Pd / C (5%), under nitrogen protection , Add 35g (0.3mol) triethylsilane at -5°C in one go, and the reaction takes about 1 hour. The cat...
Embodiment 2
[0056] Embodiment 2, preparation trans, trans-4-[4-(4-methylphenyl) cyclohexyl] cyclohexylethylene
[0057] 1. Preparation of trans, trans-4-[4-(4-methylphenyl)cyclohexyl]cyclohexylcarbonyl chloride
[0058] Trans, trans-4-[4-(4-methylphenyl)cyclohexyl]cyclohexylcarboxylic acid (0.1mol), thionyl chloride (0.15mol), benzene (100mL), the preparation method is the same as in Example 1 .
[0059] 2. Preparation of trans, trans-4-[4-(4-methylphenyl)cyclohexyl]cyclohexylcarbaldehyde
[0060] In a 500ml three-necked flask, add trans, trans-4-[4-(4-methylphenyl)cyclohexyl]cyclohexylcarbonyl chloride and 200ml dioxane prepared in the previous step, and add 2.8g Pd / C (10%), under the protection of nitrogen, add 35g (0.3mol) triethylsilane at 20°C in one batch, and the reaction takes about 1 hour. The catalyst was removed by filtration, petroleum ether was recrystallized to obtain 19.5 g of white solid, the purity of aldehyde was 98.5% (GC), the yield was 85%, DSC: C117N151I.
[006...
Embodiment 3
[0067] Example 3, trans, trans-4-[4-(3,4-difluorophenyl) cyclohexyl] cyclohexylethylene
[0068] 1. Preparation of trans, trans-4-[4-(3,4-difluorophenyl) cyclohexyl] cyclohexyl formyl chloride
[0069] Trans, trans-4-[4-(3,4-difluorophenyl)cyclohexyl]cyclohexylcarboxylic acid (0.1mol), thionyl chloride (0.15mol), benzene (100mL), the preparation method is the same as the implementation example 1.
[0070] 2. Preparation of trans, trans-4-[4-(3,4-difluorophenyl)cyclohexyl]cyclohexylcarbaldehyde
[0071] In a 500ml three-necked flask, add trans, trans-4-[4-(3,4-difluorophenyl)cyclohexyl]cyclohexylcarbonyl chloride and 200ml dichloromethane prepared in the previous step, under nitrogen protection, add 3.2g Pd / C (10%), under the protection of nitrogen, 35 g (0.3 mol) triethylsilane was added at 25° C. at one time, and the reaction took about 1.5 hours. The catalyst was removed by filtration, petroleum ether was recrystallized to obtain 25 g of white solid, the purity of aldehyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com