Novel method for synthesizing cyclopropyl bromide
A technology of cyclopropyl bromide and a new method, which is applied in the field of fine chemical intermediates, can solve problems such as environmental damage, and achieve the effects of avoiding pollution, convenient operation, and simple synthesis and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
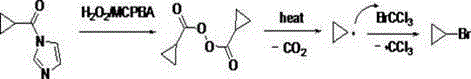
[0020] Synthesis of cyclopropionyl imidazole:
[0021] In a 250mL three-necked flask, add 150ml of dichloromethane and 34.4 grams (0.4 moles) of cyclopropanecarboxylic acid in sequence. After stirring evenly, add 64.9 grams (0.4 moles) of carbonyldiimidazole in 4-6 batches. over 30°C. After the addition was completed, the reaction was stirred at room temperature for 1 hour, and the reaction was basically complete as detected by TLC. Saturated ammonium chloride was added, and the aqueous layer was extracted once with 50 ml of dichloromethane. The organic layers were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, and evaporated to dryness to obtain liquid cyclopropionyl imidazole. This compound can be directly used in the next reaction.
[0022] Synthesis of cyclopropyl bromide:
[0023] Under nitrogen protection, in a 500mL three-necked flask, add 250ml of 1,2-dichloroethane and 79.3g (0.4mol) of trichlorobromomethane into the above ...
Embodiment 2
[0025] Synthesis of cyclopropionyl imidazole:
[0026] In a 250mL three-necked flask, add 150ml of tetrahydrofuran and 34.4g (0.4mol) of cyclopropanecarboxylic acid in sequence. After stirring evenly, add 71.3g (0.44mol) of carbonyldiimidazole in 4-6 batches. During the addition, the temperature is controlled not to exceed 30 ℃. After the addition was completed, the reaction was stirred at room temperature for 1 hour, and TLC detected that the reaction was complete. After rotary evaporation of the solvent, saturated ammonium chloride and 1,2-dichloroethane were added, washed with saturated brine, dried over anhydrous magnesium sulfate, and filtered to obtain a liquid cyclopropionyl imidazole 1,2-dichloroethane solution. This compound can be directly used in the next reaction.
[0027] Synthesis of cyclopropyl bromide:
[0028] Under the protection of nitrogen, add 220 ml of 1,2-dichloroethane and 87.2 g (0.44 mole) of trichlorobromomethane to the above-mentioned r...
Embodiment 3
[0030] Synthesis of cyclopropionyl imidazole:
[0031] In a 250mL three-necked flask, add 150ml of acetonitrile and 34.4g (0.4mol) of cyclopropanecarboxylic acid in sequence. After stirring evenly, add 64.9g (0.4mol) of carbonyldiimidazole in 4-6 batches. During the addition process, control the temperature not to exceed 30 ℃. After the addition was completed, the reaction was stirred at room temperature for 1 hour, and TLC detected that the reaction was complete. Saturated ammonium chloride was added, and the aqueous layer was extracted once with 50 ml of dichloromethane. The organic layers were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, and evaporated to dryness to obtain liquid cyclopropionyl imidazole. This compound can be directly used in the next reaction.
[0032] Synthesis of cyclopropyl bromide:
[0033] Under nitrogen protection, add 180 ml of toluene and 95.2 g (0.48 moles) of bromotrichloromethane to the above reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com