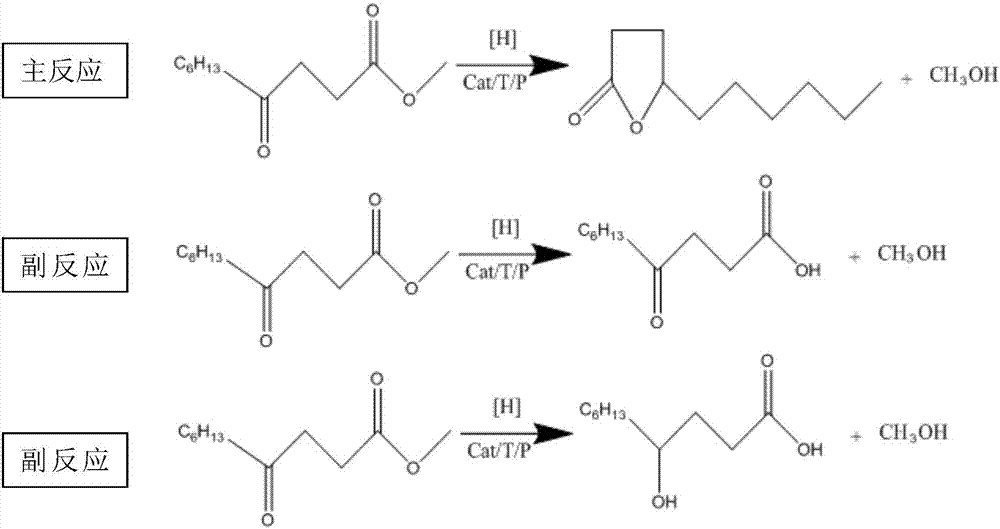
A synthetic method of chiral gamma-decalactone
A synthesis method and decanolide technology, applied in the field of essence, can solve the problems of low fermentation yield, no industrialization conditions, and high strain requirements, and achieve the effects of easy synthesis, easy production and operation, and moderate pressure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthetic method of described chiral gamma-decalactone, this synthetic method comprises the steps:
[0032] (1) Add 1.62g of concentrated sulfuric acid in 54ml of methanol, then add 0.045g of ruthenium chloride, 0.18g of BINAP, 0.9g of benzyltriethylammonium chloride, and finally add 18g of methyl 4-carbonyldecanoate to react;
[0033] (2) Transfer the reactants obtained in step (1) to the autoclave, cover the autoclave, replace the air in the autoclave with hydrogen for 3 times, then pressurize at 3.0Mpa, stir and heat up to 90°C, and pressurize the hydrogen Increase to 5.0Mpa, the reaction time is 8 hours;
[0034] (3) The reactant obtained in step (2) is neutralized, filtered, solvent recovered, and distilled to obtain the chiral γ-decalactone. The chromatographic analysis raw material content is 2.26%, and the (R) isomer is 94.39%. , (S) isomer 1.85%, calculated ee value 96.2%.
[0035] Described methyl 4-carbonyldecanoate is prepared by the method comprising ...
Embodiment 2
[0037] The synthetic method of described chiral gamma-decalactone, this synthetic method comprises the steps:
[0038] (1) Add 1.62g of concentrated sulfuric acid to 54ml of methanol, then add 0.045g of palladium chloride, 0.18g of BINAP, 0.9g of benzyltriethylammonium chloride, and finally add 18g of methyl 4-carbonyldecanoate to react;
[0039] (2) Transfer the reactants obtained in step (1) to the autoclave, cover the autoclave, replace the air in the autoclave with hydrogen for 3 times, then pressurize at 3.0Mpa, stir and heat up to 90°C, and pressurize the hydrogen Increase to 5.0Mpa, the reaction time is 8 hours;
[0040] (3) The reactant obtained in step (2) is neutralized, filtered, solvent recovered, and distilled to obtain the chiral gamma-decalactone, the chromatographic analysis raw material content is 2.6%, (R) isomer 93.33% , (S) isomer 2.27%, calculated ee value 95.3%.
[0041] Described methyl 4-carbonyldecanoate is prepared by the method comprising the follo...
Embodiment 3
[0043] The synthetic method of described chiral gamma-decalactone, this synthetic method comprises the steps:
[0044] (1) Add 1.62g concentrated sulfuric acid in 54ml methanol, then add rhodium chloride 0.045g, 0.18g BINAP, benzyltriethylammonium chloride 0.9g, finally add 4-carbonyldecanoic acid methyl ester 18g and react;
[0045] (2) Transfer the reactants obtained in step (1) to the autoclave, cover the autoclave, replace the air in the autoclave with hydrogen for 3 times, then pressurize at 3.0Mpa, stir and heat up to 90°C, and pressurize the hydrogen Increase to 5.0Mpa, the reaction time is 8 hours;
[0046] (3) The reactant obtained in step (2) is neutralized, filtered, solvent recovered, and distilled to obtain the chiral γ-decalactone. The raw material content of chromatographic analysis is 1.6%, and the (R) isomer is 93.93%. , (S) isomer 2.43%, calculated ee value 95.0%.
[0047] Described methyl 4-carbonyldecanoate is prepared by the method comprising the followi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com