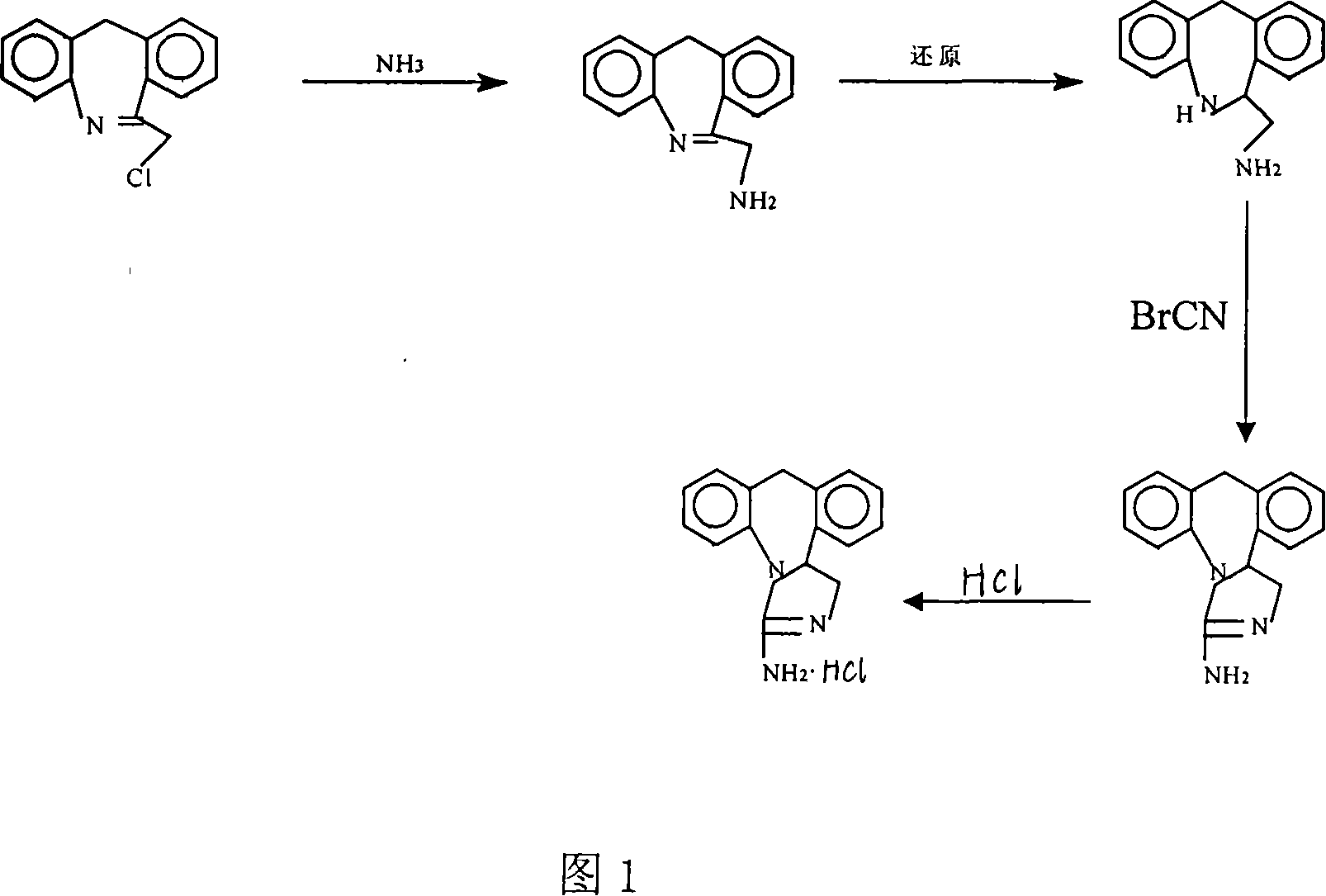
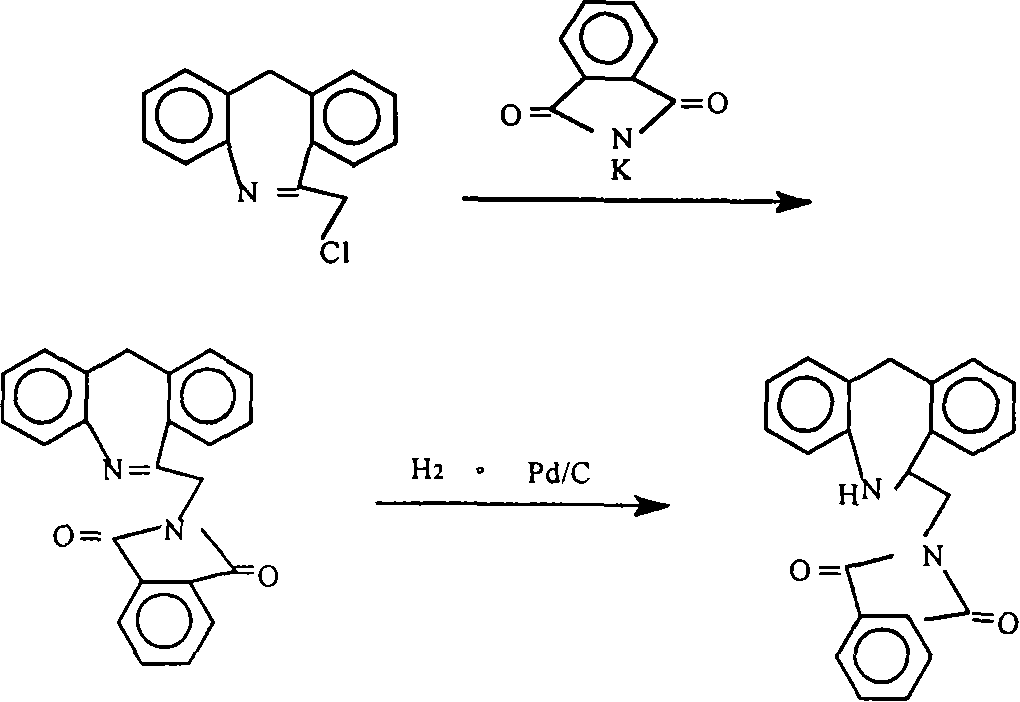
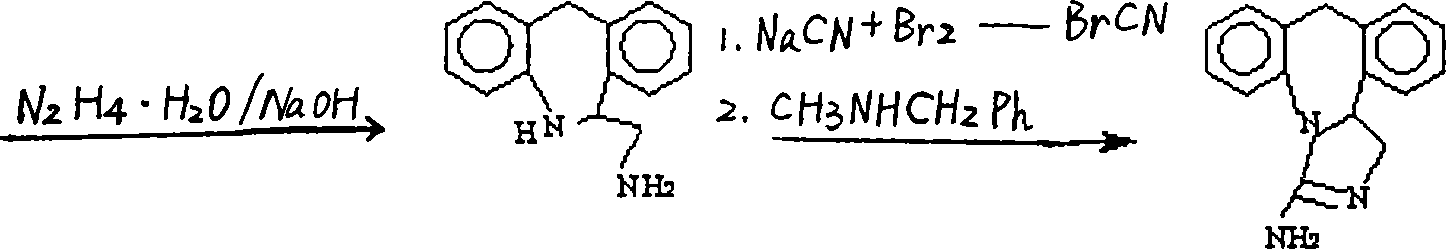Chemical synthesis method for epinastine
A chemical synthesis, epinastine technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problems that are not suitable for industrial production, and achieve high reaction yield, less by-products, and simple preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Amination reaction
[0022] In a 500ML reactor, put 20 grams of starting material 6-chloromethyl-11-dihydro-dibenzo[b, e]azepine, 300 milliliters of absolute ethanol, feed saturated ammonia gas,- React at 20°C for 24 hours, take the reaction solution for liquid phase detection, when the product content is greater than 95%, stop the reaction, add water and dichloromethane for extraction, and recover the solvent to obtain the product 6-aminomethyl-11-dihydro-diphenyl And [b, e] azepine 17.5 g (HPLC.99%, yield 95%).
[0023] (2) Reduction reaction
[0024] In a 500ML reactor, put 20 grams of raw materials 6-aminomethyl-11-dihydro-dibenzo[b, e]azepine, 300 milliliters of methanol, 6 grams of potassium borohydride, and react at 0°C for 36 hour, take the reaction liquid liquid phase detection, when the raw material is less than 1%, the reaction ends. Extracted with dichloromethane, washed with water, dried over anhydrous sodium sulfate, and recovered the solvent to obt...
Embodiment 2
[0029] (1) Amination reaction
[0030] In a 500ML reactor, put 30 grams of starting materials 6-chloromethyl-11-dihydro-dibenzo[b, e]azepine, 400 milliliters of methanol, and feed 20 ml of concentrated ammonia water, at 40°C After reacting for 10 hours, take the liquid phase of the reaction solution for detection. When the product content is greater than 95%, stop the reaction, add water and dichloromethane for extraction, and recover the solvent to obtain the product 6-aminomethyl-11-dihydro-dibenzo[b, e] 26 grams of azepines (HPLC.99%, yield 94.5%).
[0031] (2) Reduction reaction
[0032] In a 500ML reactor, put 30 grams of raw materials 6-aminomethyl-11-dihydro-dibenzo[b, e]azepine, 300 milliliters of DMF, 5 grams of 5% palladium carbon, and feed hydrogen at a pressure of 0 MPa , reacted at 80° C. for 24 hours, took the liquid phase of the reaction solution for detection, and when the raw material was less than 1%, the reaction ended. Extract with dichloromethane, wash ...
Embodiment 3
[0037] (1) Amination reaction
[0038] In the 500ML reactor, drop into starting material 6-chloromethyl-11-dihydro-dibenzo[b, e] 30 grams of azepines, 400 milliliters of ethylene glycol monomethyl ether, pass into liquid ammonia, React at 80°C for 2 hours, take the reaction liquid for liquid phase detection, when the product content is greater than 95%, stop the reaction, add water and dichloromethane for extraction, and recover the solvent to obtain the product 6-aminomethyl-11-dihydro-diphenyl And [b, e] azepine 27 g (HPLC.99%, yield 95%).
[0039] (2) Reduction reaction
[0040] In a 500ML reactor, put 20 grams of raw materials 6-aminomethyl-11-dihydro-dibenzo[b, e]azepine, 300 milliliters of absolute ethanol, and 4.5 grams of sodium borohydride at 60°C After reacting for 4 hours, take the liquid phase of the reaction solution for detection, and when the raw material is less than 1%, the reaction is over. Extracted with dichloromethane, washed with water, dried over anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com