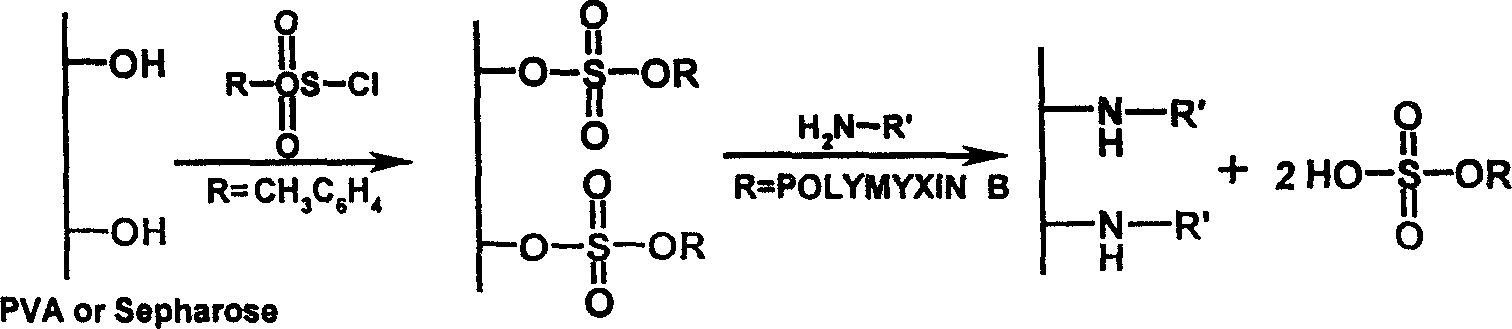Endotoxin adsorptive material, preparing and use thereof
An adsorption material and endotoxin technology, applied in the field of biomedical materials, can solve the problems that cannot be used to treat endotoxemia, poisoning, and intravenous injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation method of agarose gel beads-polymyxin endotoxin adsorbent
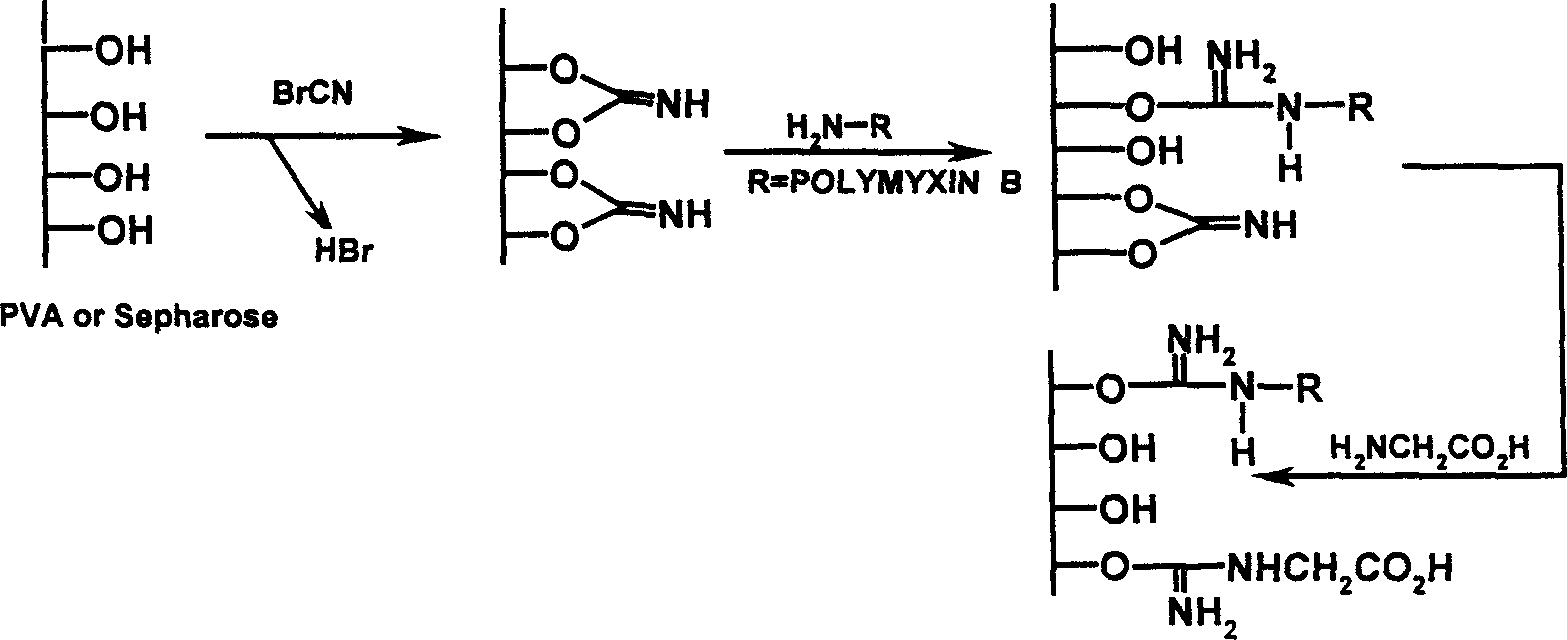
[0022] Dried Sepharose CL-4B 50mL, washed 3 times with 3 liters of 1M deoxycholic acid, washed 3 times with 3 liters of sterile pyrogen-free water, pH 7.0, and drained. Use the following two methods (1) and (2) to activate and immobilize:
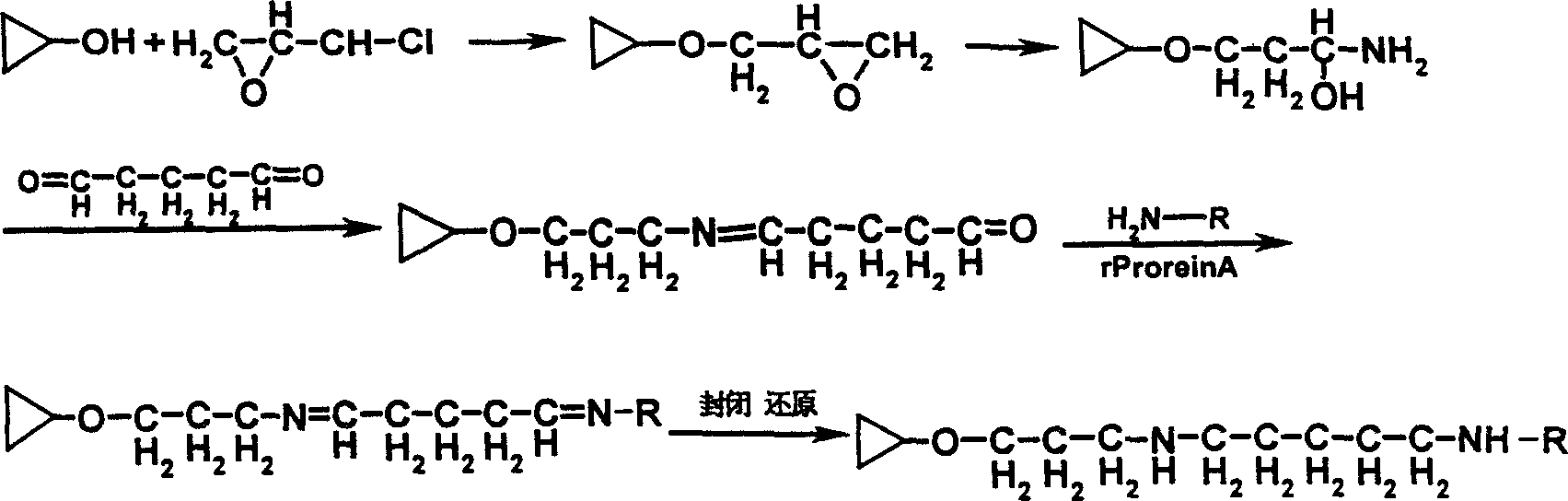
[0023] (1) Soak in 200mL epichlorohydrin (0.1M sodium hydroxide solution), shake and react at 45°C for 5 hours, rinse with 3 liters of sterile pyrogen-free water for injection to pH 7.0, filter and drain. Add 100 mL of ammonia water, shake and react at 45°C for 5 hours, add 5 mL of 25% glutaraldehyde, react at 45°C for 2 hours, wash with sterile pyrogen-free water, filter and drain. Add 200 mL of solution A (350 mg / mL polymyxin, 0.5 M sodium chloride, 0.1 M sodium bicarbonate), and react at 20° C. overnight. 10 mL of 1M glycine and 2 g of sodium borohydride were added, and the reaction was continued for 2 hours. Filter and drain, 3 liters of ...
Embodiment 2
[0027] Embodiment 2: the preparation method of PVA-polymyxin endotoxin adsorbent
[0028] Dried PVA 50mL, washed 3 times with 3 liters of 1M deoxycholic acid, washed 3 times with 3 liters of sterile pyrogen-free water, pH 7.0, and drained. Use the following two methods (1) and (2) to activate and immobilize:
[0029] (1) Soak in 200mL epichlorohydrin (0.1M sodium hydroxide solution), shake and react at 55°C for 2 hours, rinse with 3 liters of sterile pyrogen-free water for injection to pH 7.0, filter and drain. Add 100 mL of ammonia water, shake and react at 55°C for 2 hours, add 5 mL of 25% glutaraldehyde, react at 55°C for 2 hours, rinse with sterile pyrogen-free water, filter and drain. Add 200 mL of solution A (350 mg / mL polymyxin, 0.5 M sodium chloride, 0.1 M sodium bicarbonate) and react at 35° C. for 6 hours. 10 mL of 1M glycine and 2 g of sodium borohydride were added, and the reaction was continued for 1 hour. Filter and drain, 3 liters of sterile pyrogen-free wate...
Embodiment 3
[0040] Embodiment 3: the use method of endotoxin adsorption material:
[0041] (1) Mammalian whole blood perfusion:
[0042] Material 4 was packed into a column (inner diameter 4 cm, height 4 cm) for hemoperfusion.
[0043] The femoral artery and femoral vein of the mixed adult dog (weight 7-9kg) were connected to the perfusion device through a tube pump system, the blood flow rate was controlled at 50mL / min, and the dog was under anesthesia. 15 minutes after the start of perfusion, the normal saline containing E. coli 0111:B4 0.01% (0.5mg / kg body weight) lipopolysaccharide solution was titrated for 60 minutes and injected into the forefoot, and the amount of heparin in the perfusion test was controlled at 100 units / kg body weight / hour , perfusion continued for 2 hours. One in 10 dogs died after 24 hours (mortality 10%), compared to 7 and 9 deaths in the control experiments of Sepharose CL-4B and PVAC treated dogs respectively (mortality 70% and 90%). The other 5 dogs that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com