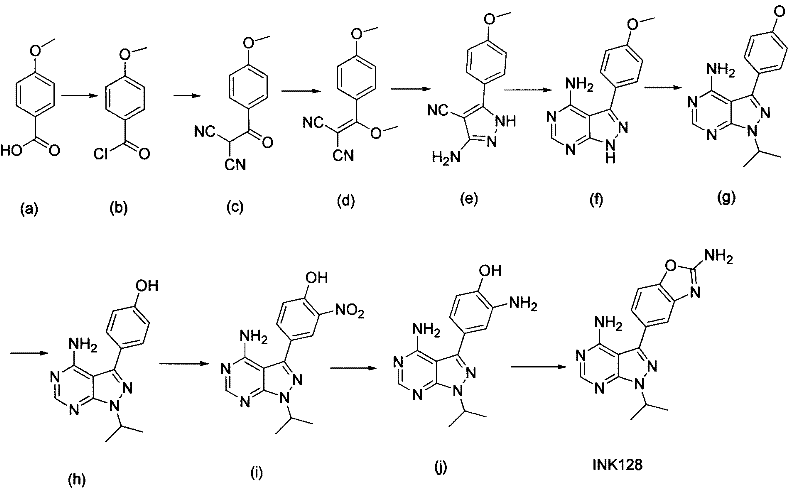
New synthesis process of new antineoplastic drug INK128
A technology of a process and a synthetic route, applied in the field of synthesis of new anti-tumor drugs, can solve the problems of non-response or drug resistance of cancer patients, threatening human life and health, and treatment failure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] Preparation of compound (b):
[0013] Dissolve 152 g (1 mole) of starting material (a) in 1.5 liters of dichloromethane, add 5 milliliters of DMF and then dropwise add 254 g (2 moles) of oxalyl chloride. Under ice-water cooling, the dropwise temperature rises to room temperature and stirs for 3 hours. , spin-dried to obtain 171 g of acid chloride (yield 100%), which was directly used in the next step without purification.
[0014] Preparation of compound (c):
[0015] Dissolve 66 grams (1 mole) of propanedinitrile in 660 milliliters of DMF, cool to 0 degrees, add 44 grams (1.1 moles) of sodium hydrogen (60% purity) in batches, react at room temperature for 1 hour after the addition, and cool in an ice-salt bath Add the acid chloride obtained in the step dropwise, control the temperature below 5°C, and react overnight at room temperature after completion, the reaction is complete as detected by TLC, the reaction solution is poured into 1 liter of ice water, extracted th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com