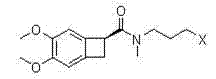
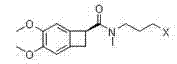
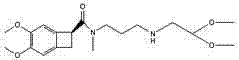
One group of novel benzene cyclobutane compounds and application of novel benzene cyclobutane compounds in chemical synthesis
A technology of benzocyclobutane and compound, applied to benzocyclobutane compound and its application field in the preparation of ivabradine, can solve the problem of high preparation cost of ivabradine, high preparation difficulty, complicated operation, etc. problem, to achieve the effect of high product yield, low cost and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Compound III is (1S)-[N-(3-(2,2-dimethoxyethylamine)-propyl)-N-methyl]carbamoyl-4,5-dimethoxy Preparation of phenylbenzocyclobutane
[0050] Dissolve 35 mL of aminoacetaldehyde dimethyl acetal and 50 mL of triethylamine in 300 mL of dichloromethane, place the reaction bottle in an ice bath, and dissolve 89.3 g of (1S)-[N-(3-chloropropyl)-N -Methyl]carbamoyl-4,5-dimethoxybenzocyclobutane was dissolved in 450mL of dichloromethane to form a solution, the solution was dropped into the aforementioned reaction solution placed in an ice bath, and stirred at room temperature , saturated with 300 mL NaHCO 3 The organic layer was washed twice, and then washed twice with 300 mL of water. The organic layer was collected, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 98.9 g of a brownish-yellow oily substance, Compound III, with a yield of 90%.
Embodiment 2
[0051] Example 2: Compounds Namely (1S)-[N-(3-(2,2-dimethoxyethylamine)-propyl)-N-methyl]aminomethyl-4,5-dimethoxybenzocyclobutane preparation of
[0052] Pass nitrogen protection into the reaction flask containing 73.3 g of compound III prepared in Example 1, add 350 mL of anhydrous tetrahydrofuran, stir to dissolve, add 10.0 g of lithium tetrahydrogen to the reaction solution in batches under ice bath, and complete the addition , stirred at room temperature for 5 hours, the raw material reaction of the sampling spot plate was complete, slowly added 50 mL of water dropwise to the reaction solution to quench the reaction, added 500 mL of ethyl acetate to the reaction solution, then added anhydrous sodium sulfate, stirred and dried for 2 hours, filtered In addition to desiccant, the filtrate was concentrated under reduced pressure to obtain the residue that is the compound 61.3 g, yield 87%.
Embodiment 3
[0053] Example 3: Compounds That is, N-(3-(N-(((S)-1,2-dihydro-4,5-dimethoxybenzocyclobutane-2-yl)methyl)-N-formamide)propane Preparation of -N-(2,2-dimethoxyethyl)-2-(3,4-dimethoxyphenyl)acetamide
[0054] Add 400 mL of dichloromethane into a 1 L reaction flask, add 39.2 g of 3,4-dimethoxyphenylacetic acid while stirring, cool down to 0°C~5°C in an ice bath, and start adding 28.5 g of thionyl chloride dropwise. Control the rate of addition so that the temperature of the reaction solution does not exceed 10°C, remove the ice-water bath after the addition, stir and react at room temperature for 2 hours, evaporate the solvent to dryness under reduced pressure to obtain a brown oil, add 200 mL of dichloromethane to dissolve, and set aside;
[0055] 350mL contains the compound that 35.2g embodiment 2 prepares Adjust the pH of the dichloromethane solution to 8~9 with triethylamine, add the prepared dichloromethane solution containing brown oil dropwise, control the rate of addi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com