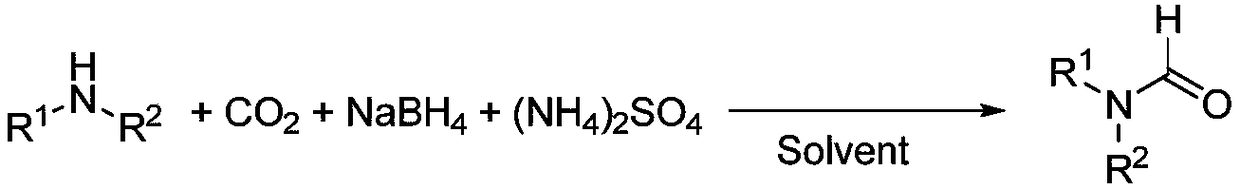
N-formylation synthesis method taking CO2 as carbon source under mild condition
A technology of chemical synthesis and conditions, applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carboxylic acid amides, etc., can solve the problems of development restriction, low utilization rate, high price, etc. The effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
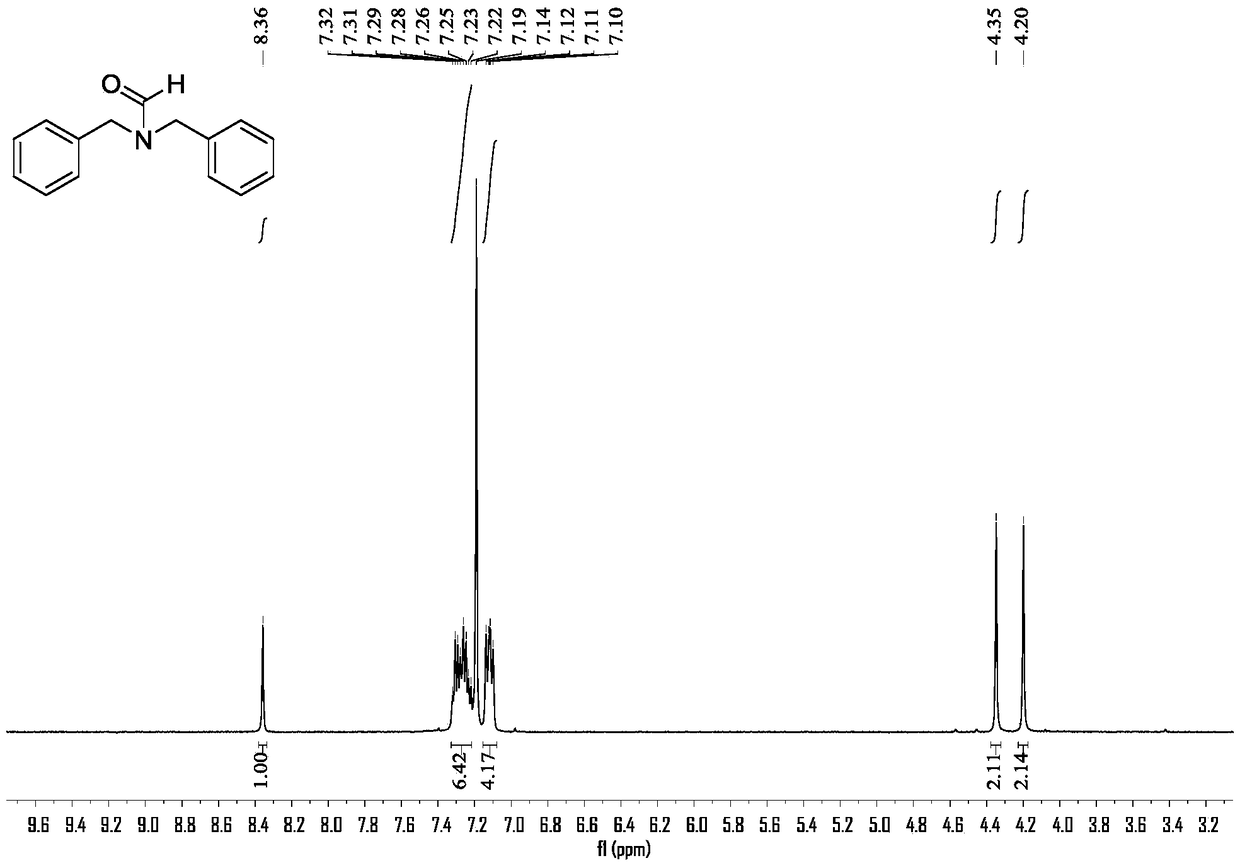
[0025] The preparation method of N,N-dibenzylformamide:
[0026] (1) Under the protection of nitrogen, sodium borohydride (0.019g, 0.5mmol) and ammonium sulfate (0.071g, 0.5mmol) were added to anhydrous THF (2mL), and stirred at 25°C for 1h. Then, nitrogen gas was replaced by carbon dioxide gas.
[0027] (2) Dibenzylamine (0.099g, 0.5mmol) was dissolved in anhydrous THF (3mL), and added dropwise to the reaction system under an atmosphere of carbon dioxide. After 0.75h, the dropwise addition was completed. GC monitors the reaction, and after the reaction of the raw material amine is complete, the reaction is terminated.
[0028] (3) Using ethyl acetate and petroleum ether (volume ratio 1:1) as developing solvents, the product was separated by plate chromatography. The silica gel was rinsed with a mixed solvent of dichloromethane and methanol (10:1 by volume). The eluate was collected, the solvent was removed by rotary evaporation, and vacuum-dried to obtain 0.087 g of the co...
Embodiment 2
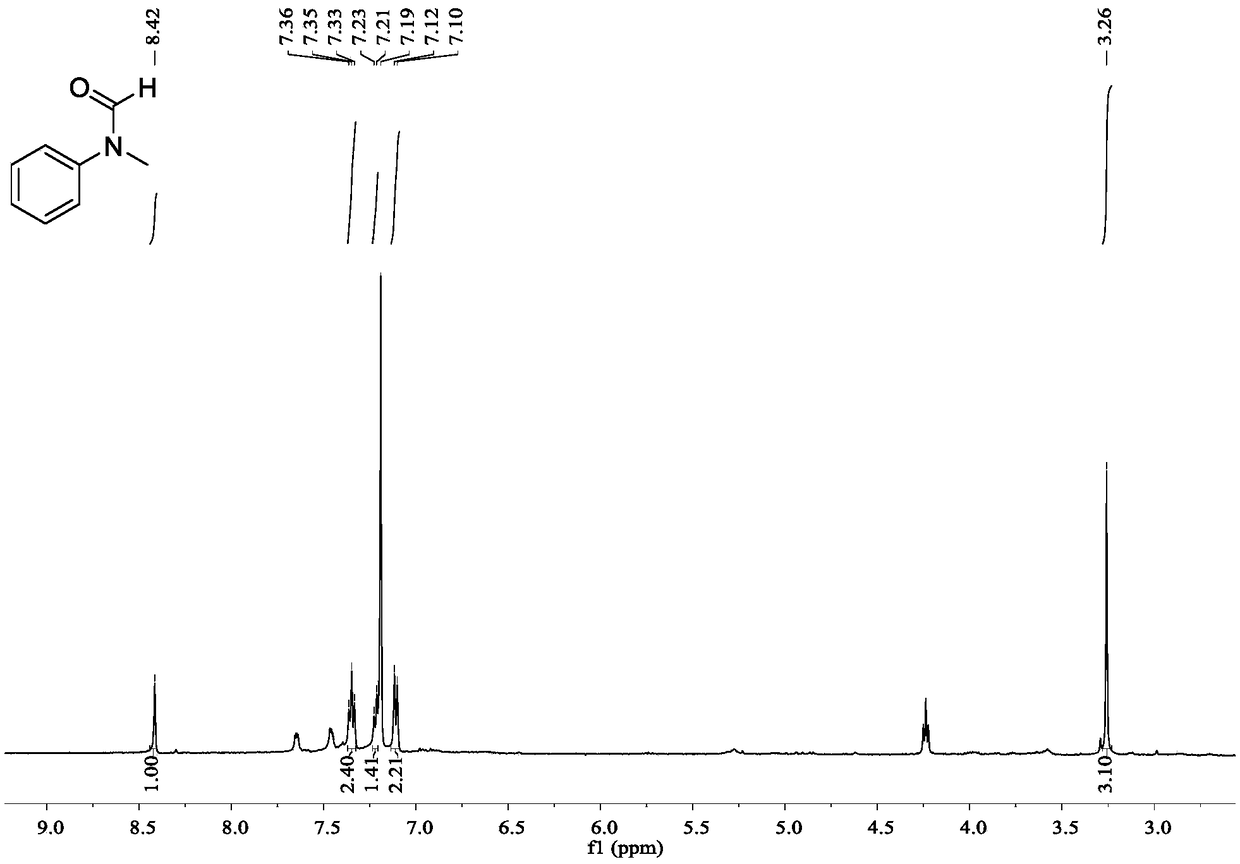
[0030] The preparation method of N-methyl-N-phenyl formamide:
[0031] (1) Under nitrogen protection, sodium borohydride (0.076g, 2mmol) and ammonium sulfate (0.28g, 2mmol) were added to anhydrous THF (2mL), and stirred at 25°C for 1h. Then, nitrogen gas was replaced by carbon dioxide gas.
[0032] (2) N-Methylaniline (0.21g, 2mmol) was dissolved in anhydrous THF (3ml), and added dropwise to the reaction system under an atmosphere of carbon dioxide. After 0.5h, the dropwise addition was completed. GC monitors the reaction, and after the reaction of the raw material amine is complete, the reaction is terminated.
[0033] (3) Using ethyl acetate and petroleum ether (volume ratio 1:4) as the developing solvent, the product was separated by plate chromatography. Utilize the mixed solvent of dichloromethane and methanol (10:1 by volume) to rinse the silica gel, collect the eluate, remove the solvent by rotary evaporation, and dry in vacuo to obtain 0.127 g of the corresponding fo...
Embodiment 3
[0035] Under the condition of adding catalyst, the preparation method of N,N-dibenzylformamide:
[0036] (1) Under nitrogen protection, sodium borohydride (0.019g, 0.5mmol), ammonium sulfate (0.071g, 0.5mmol) and copper chloride dihydrate (0.002g, 0.012mmol) were added to anhydrous THF (2mL) , Stir the reaction at 25°C for 1h. Then, nitrogen gas was replaced by carbon dioxide gas.
[0037] (2) Dibenzylamine (0.099g, 0.5mmol) was dissolved in anhydrous THF (3mL), and added dropwise to the reaction system under an atmosphere of carbon dioxide. After 0.67h, the dropwise addition was completed. GC monitors the reaction, and after the reaction of the raw material amine is complete, the reaction is terminated.
[0038] (3) Using ethyl acetate and petroleum ether (volume ratio 1:1) as developing solvents, the product was separated by plate chromatography. The silica gel was rinsed with a mixed solvent of dichloromethane and methanol (10:1 by volume). The eluate was collected, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com