Synthetic method of ceftriaxone sodium crude salt
A technology of ceftriaxone sodium and a synthesis method, applied in the field of chemical synthesis, can solve the problems of high raw material cost, long reaction time, low yield and the like, and achieve the advantages of improving product purity, avoiding side reactions, and improving product yield and purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
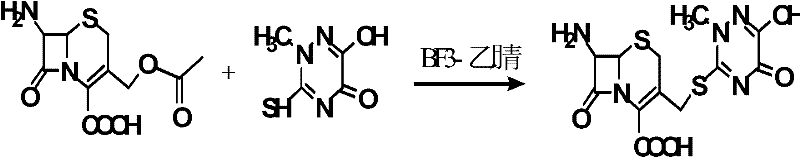
[0027] (1) Synthesis of 7-ACT:
[0028] According to the preparation steps of the comparative example, after adding purified water, add 16 g of solid-phase cephalosporin ester hydrolase, constantly adjust the pH to 2.0 with 5% (volume fraction) of ammonia water, until the pH is stable, and the crystallization procedure is also the same as compared instance. After drying, 23.79 g of dry product was obtained, the purity (HPLC) was 99.25%, and the molar yield was 85% [n(7-ACT) / n(7-ACA)×100%].
[0029] (2) the synthesis of ceftriaxone sodium coarse salt:
[0030] According to the preparation steps of the comparative example, add solvent dichloromethane 100mL, 7-ACT 22g (58.67mmol) and AE-active ester 21g (59mmol) in the three-necked flask, react at 0 ℃ for 2h, add triethylamine until the reaction liquid is Alkaline and then react for 7h. Then add flocculant 0.22g, stir at low speed for 10min, and filter. To the filtrate was added 78 mL of an aqueous solution containing 115 g (...
Embodiment 2
[0032] (1) Synthesis of 7-ACT:
[0033] According to the preparation steps of the comparative example, after adding purified water, add 18 g of solid-phase cephalosporin ester hydrolase, constantly adjust the pH to 2.0 with 5% (volume fraction) of ammonia water until the pH is stable, and the crystallization procedure is also the same as compared instance. After drying, 24.64 g of dry product was obtained, the purity (HPLC) was 99.25%, and the molar yield was 88% [n(7-ACT) / n(7-ACA)×100%].
[0034] (2) the synthesis of ceftriaxone sodium coarse salt:
[0035] According to the preparation steps of the comparative example, add solvent dichloromethane 100mL, 7-ACT 22g (58.67mmol) and AE-active ester 21g (59mmol) in the three-necked flask, react at 0 ℃ for 2h, add triethylamine until the reaction liquid is Alkaline and then react for 7h. Then add 0.30 g of flocculant, stir at low speed for 10 min, and filter. To the filtrate was added 78 mL of an aqueous solution containing 115...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com