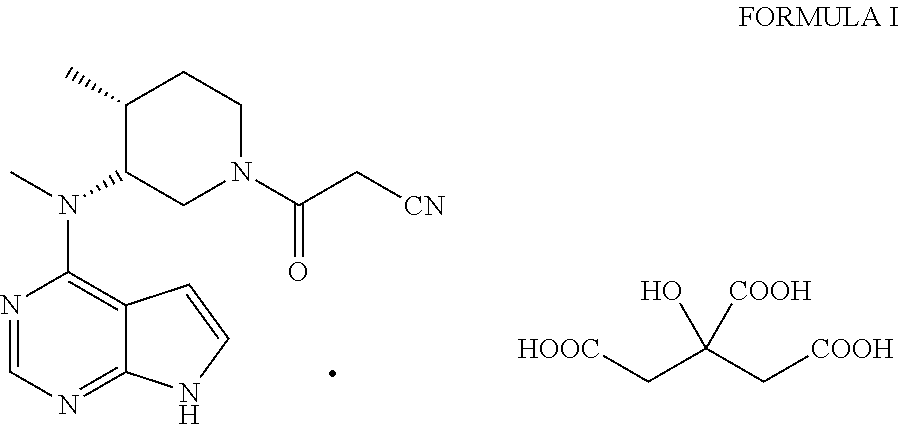
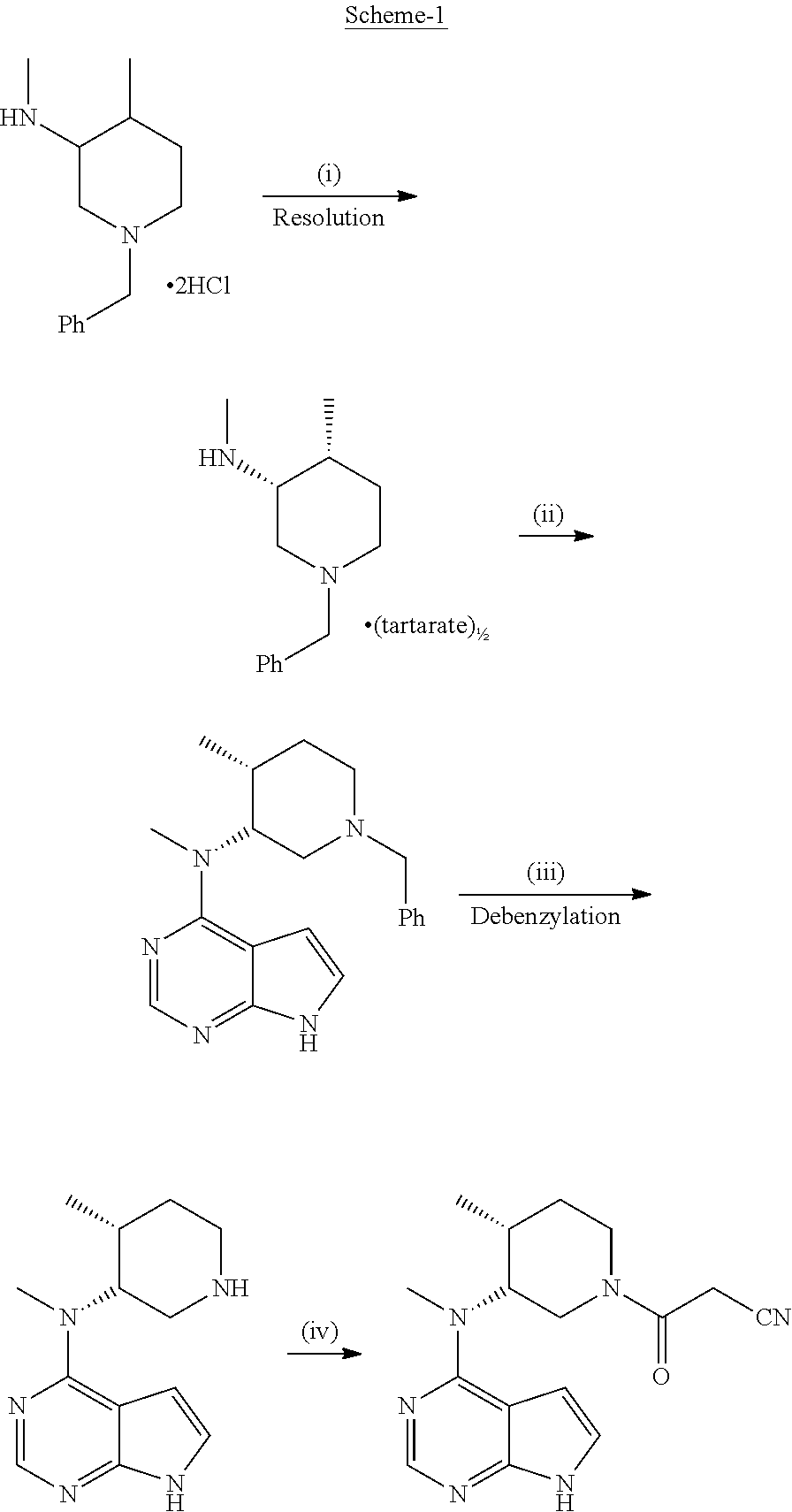
Process for the preparation of (3r,4r)-(1-benzyl-4-methylpiperidin-3-yl)-methylamine
a technology of piperidin and methylamine, which is applied in the field of process for the preparation of (3r, 4r)(1benzyl-4-methylpiperidin3yl)methylamine, can solve the problems of high cost of reagents, difficult commercial use, and complicated synthesis, and achieves improved and efficient results. , the effect of improving the yield over all
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example-1
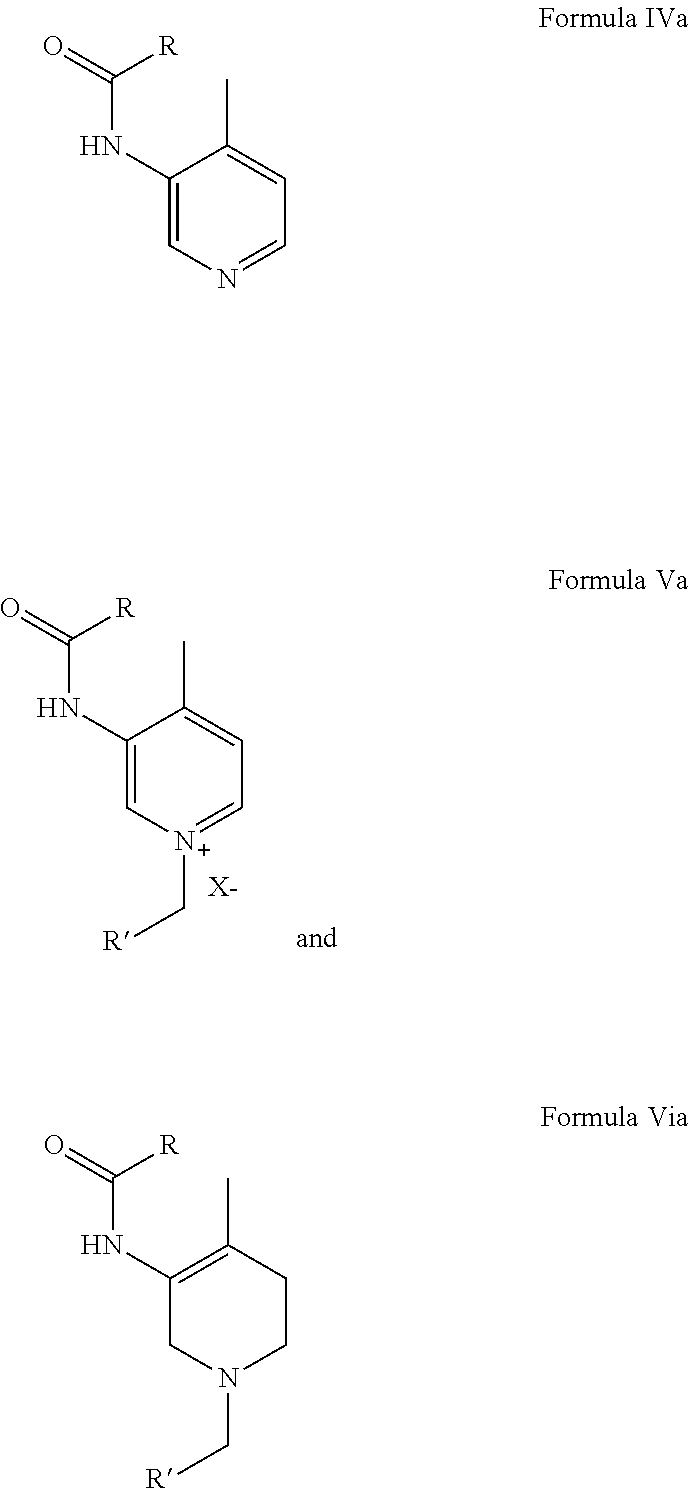
Preparation of N-(4-methylpyridin-3-yl)-acetamide from 3-Amino-4-methyl pyridine
[0073]3-Amino-4-methyl pyridine (200 gm) and Acetic acid (600 mL) were charged in a 2 L 4-neck round bottom flask with an overhead stirrer and stirred for 15 minutes at RT. Acetic anhydride (284 gm / 263 mL) or Acetyl chloride (174 gm) was added drop wise within 1-2 h at that temperature. The reaction mass was then stirred at RT for 8-10 h. After the completion of the reaction as monitored by TLC, HPLC; acetic acid was distilled out under vacuum. Methanol (1 L) was then added to the reaction mixture and the pH of the reaction mixture was maintained around 10-12 by liq. Ammonia. Methanol was distilled out completely under vacuum at 50° C. to 55° C. The product was then extracted with MDC (1 L) to get the pure product. Yield: 98% w / w; HPLC Purity: 98%.
example-2
Preparation of N-(4-methylpyridin-3-yl)-acetamide from 3-Amino-4-methyl pyridine
[0074]3-Amino-4-methyl pyridine (200 gm) and Acetic anhydride (284 gm / 263 mL) in a 2 L 4-neck round bottom flask with an overhead stirrer were stirred for 15 minutes at RT. The stirring was continued at RT for 1-3 h. After the completion of the reaction as monitored by TLC, Methanol (1 L) was added to the reaction mixture and the pH of the reaction mixture was maintained around 10-12 by liq. Ammonia. Methanol was distilled out completely under vacuum at 50° C. to 55° C. Extraction with MDC (1 L) gave pure product. Yield: 98% w / w; HPLC Purity: 98%.
example-3
Preparation of N-(4-methylpyridinium-3-yl)-acetamide acetate from 3-amino-4-methyl pyridine
[0075]3-Amino-4-methyl pyridine (200 gm), Acetic anhydride (284 gm / 263 mL) or Acetyl chloride (174 gm) and MDC (1 L) in a 2 L 4-neck round bottom flask with an overhead stirrer were stirred for 15 minuets at RT. The reaction mass was stirred at RT for 8-10 h. Completion of the reaction was monitored by TLC, HPLC. Extraction with MDC (1 L) gave pure product. Yield: 98% w / w; HPLC Purity: 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com