Bisamide type ultra-long-life room-temperature phosphorescent compound as well as preparation method and application thereof
An ultra-long-life, room-temperature phosphorescence technology, used in security and biological imaging applications, preparation of bisamide compounds from carbazole and diacyl chloride compounds, in the field of anti-counterfeiting, can solve problems such as limited applications, and achieve easy post-processing , The synthesis steps are simple, and the effect of the spin-orbit coupling effect is enhanced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
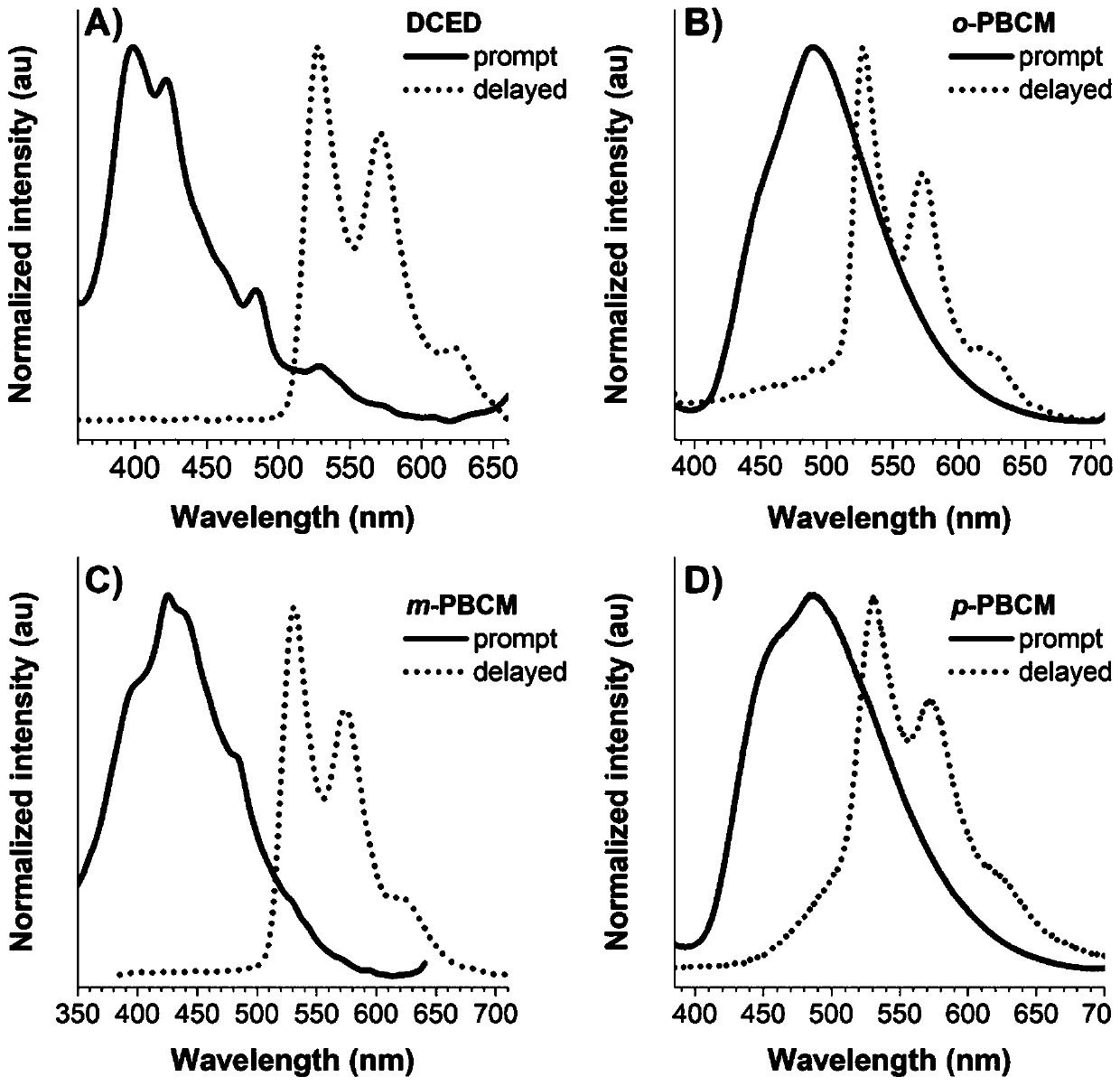
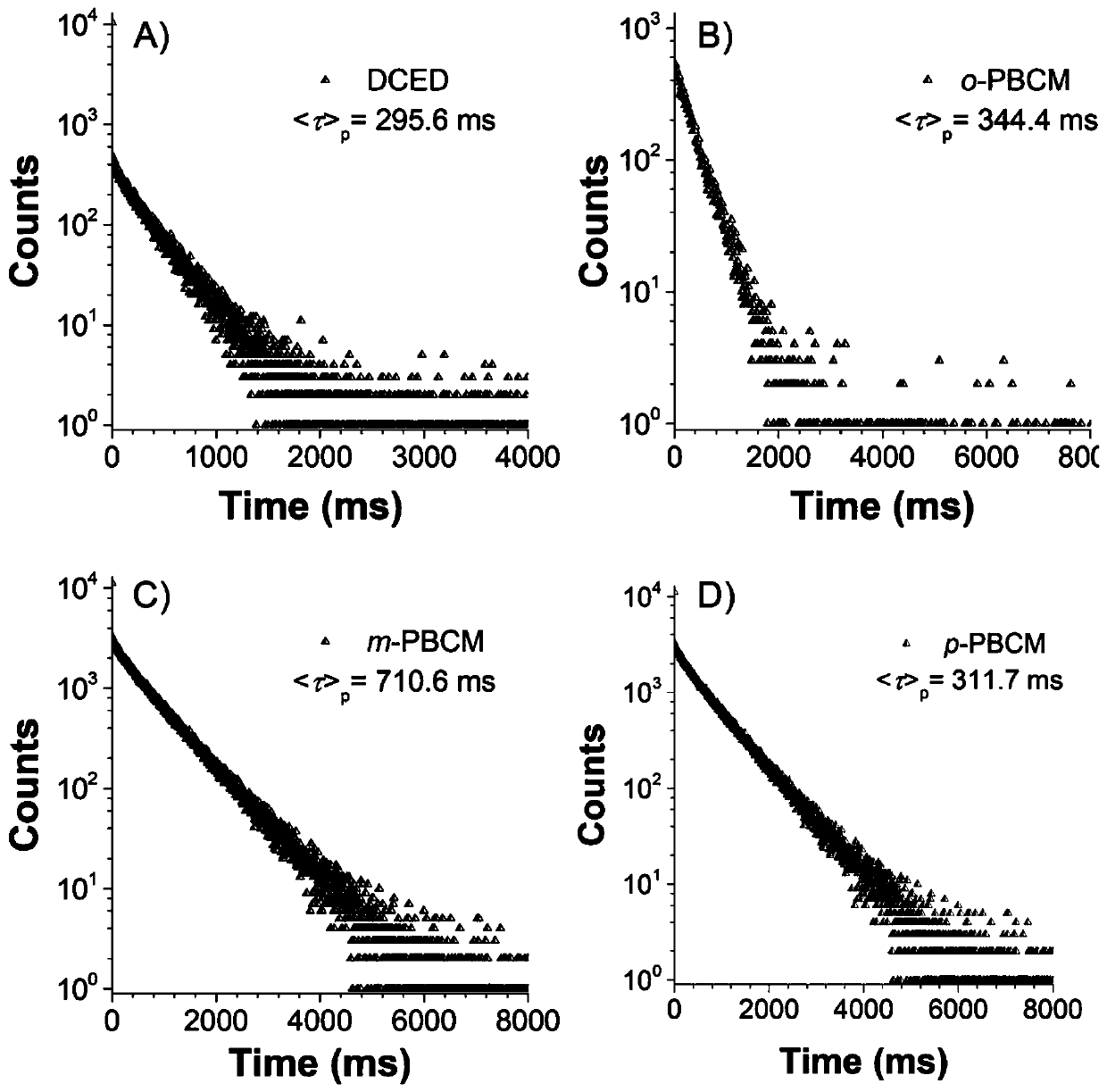
[0040] Preparation of compound DCED
[0041]
[0042] Add 3.83g of sodium hydride (95.59mmol, 60% dispersed in kerosene) in a 250ml two-necked round-bottomed flask, dissolve 8g of carbazole (47.84mmol) in 120ml of redistilled tetrahydrofuran solution and add it to the flask, after stirring for about half an hour , add 6.07ml (71.77mmol) oxalyl chloride reagent dropwise. The reaction was carried out overnight at room temperature, and the progress of the reaction was monitored by silica gel chromatography (TLC). After the reaction was completed, distilled water was added to the flask to quench, and the liquid was extracted and separated with dichloromethane / saturated brine. The organic layer was dried over anhydrous sodium sulfate, filtered with suction, and the organic solvent was removed by rotary evaporation. The crude product was separated and purified by silica gel column chromatography (volume ratio of eluent: DCM / PE=1:10-1:2) to obtain 3.95 g of white solid powder wit...
Embodiment 2
[0047] Preparation of compound o-PBCM
[0048]
[0049] Concrete synthetic steps are similar to DCED synthetic steps, add 3.83g sodium hydride (95.59mmol, 60% are dispersed in kerosene) in 250ml two-necked round-bottomed flasks, 8g carbazole (47.84mmol) are dissolved in 120ml redistilled tetrahydrofuran solution and Add it to the flask, stir for about half an hour, then add 10.54 mL (98%, 71.77 mmol) of phthaloyl chloride reagent dropwise. The reaction was carried out overnight at room temperature, and the progress of the reaction was monitored by silica gel chromatography (TLC). After the reaction was completed, distilled water was added to the flask to quench, and the liquid was extracted and separated with dichloromethane / saturated brine. The organic layer was dried over anhydrous sodium sulfate, filtered with suction, and the organic solvent was removed by rotary evaporation. The crude product was separated and purified by silica gel column chromatography (volume ratio...
Embodiment 3
[0054] Preparation of compound m-PBCM
[0055]
[0056] Add 2.39g of sodium hydride (59.80mmol, 60% dispersed in kerosene) in a 500ml two-necked round bottom flask, dissolve 5g of carbazole (29.90mmol) in 120ml of redistilled tetrahydrofuran solution and add it to the flask, after stirring for about half an hour , 9.11g (44.85mmol) of isophthaloyl chloride was dissolved in 70ml of redistilled tetrahydrofuran solution and added dropwise to the reaction system. The reaction was carried out overnight at room temperature, and the progress of the reaction was monitored by silica gel chromatography (TLC). After the reaction was completed, distilled water was added to the flask to quench, and the liquid was extracted and separated with dichloromethane / saturated brine. The organic layer was dried over anhydrous sodium sulfate, filtered with suction, and the organic solvent was removed by rotary evaporation. The crude product was separated and purified by silica gel column chromato...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com