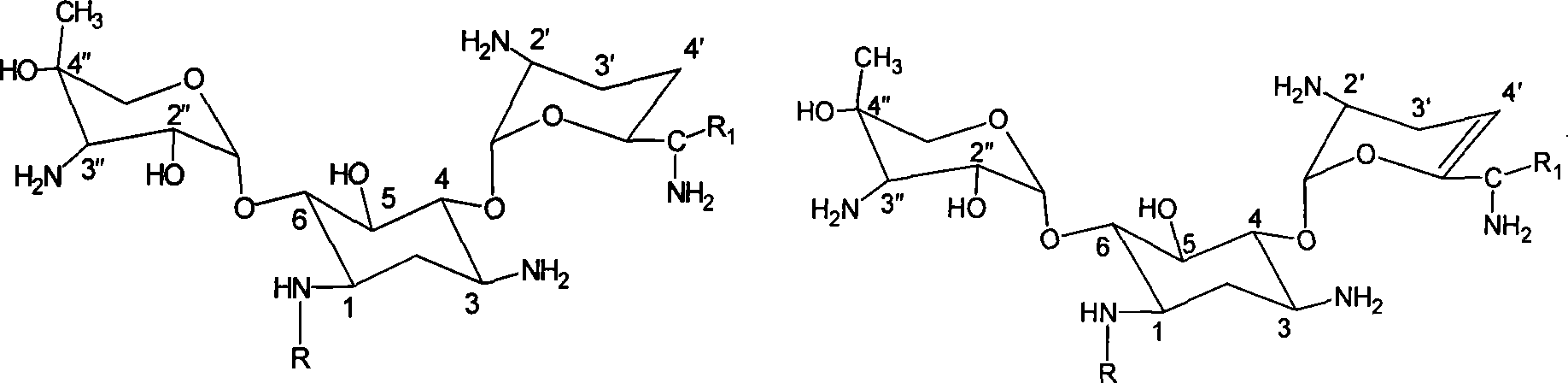
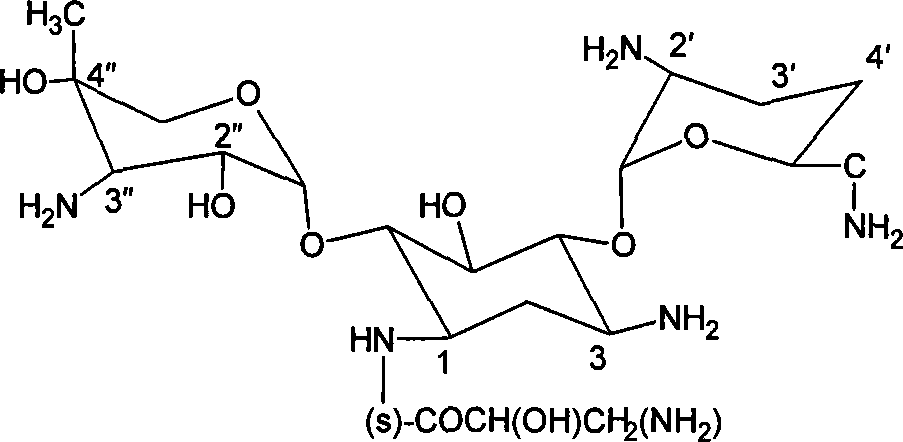
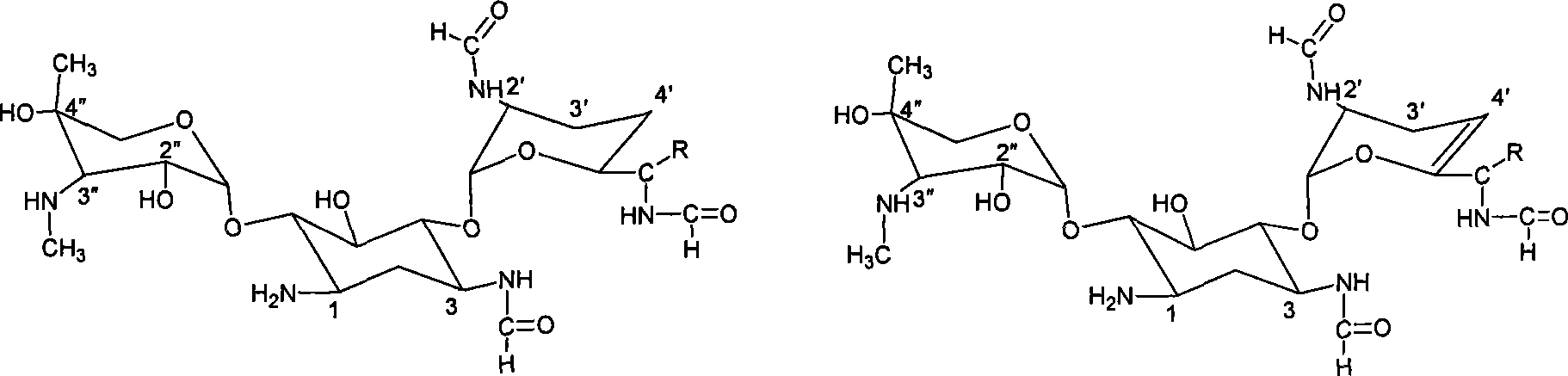
Aminoglycoside antibiotics
A technology of aminoglycosides and antibiotics, applied in the direction of amino sugar, etc., can solve the problem of not being able to cover the invention concept
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Synthesis of 1-N-(S)-3-amino-2-hydroxypropionyl gentamicin C1a 1) 3,2 ', 6'-three-N-formyl gentamicin C1a synthesis
[0128] 25 grams of gentamicin C1a (GMC1a) was dissolved with 500 mL of dimethyl sulfoxide (DMSO) under stirring, cooled to room temperature, 100 mL of dichloromethane and 32 grams of cobalt acetate tetrahydrate were added, stirred and dissolved at room temperature and complexed. Add 40 g of 2-formylmercaptothiazole in batches, and react for 1 hour under stirring. Add 500mL of 5°C cold water to the reaction solution and mix evenly, separate the liquids, add 5 grams of activated carbon to the upper layer and stir for 20 minutes to filter, and the filtrate is extracted with 1 / 10 of the volume of the filtrate with dichloromethane, and extracted twice.
[0129] The upper aqueous phase was dynamically adsorbed by an 800mL HD-2 resin (ammonium type) column, washed with water to remove DMSO, and then eluted with 0.4N ammonia water. When the pH reache...
Embodiment 2
[0136] Example 2: Synthesis of 1-N-(S)-3-amino-2-hydroxypropionyl-3″-N-demethylgentamycin C1a
[0137] Add 20 mL of water and 100 mL of DMSO to 5 grams of 3″-N-demethylgentamycin C1a, stir to dissolve, add 10 grams of zinc acetate to dissolve, and add 8.5 grams of 2-formylmercaptothiazole in batches at room temperature to react. Reaction Add 150mL cold water to the solution, mix evenly, and filter. The filtrate is dynamically adsorbed with weakly acidic cationic resin D151, washed with water, and eluted with 0.4N ammonia water. When the pH is 9.5, it starts to be collected in sections. The same components are combined and evaporated to dryness under reduced pressure to obtain Solid 3.5 g.
[0138] The above solid was subjected to 1-N acylation reaction and purified by the method of step 2) in Example 1 to obtain 1-N-(S)-3-amino-2-hydroxypropionyl-3″-N-demethylated Qingda Mycin C1a base 1.8 g.
[0139] Take 1.5 grams of the above-mentioned solids and dissolve them in 30 mL of...
Embodiment 3
[0140] Example 3: Synthesis of 1-N-(S)-3-amino-2-hydroxypropionyl-3″-N-demethylgentamycin C1a
[0141] 8 g of 1-N-(S)-3-amino-2-hydroxypropionyl gentamicin C1a sulfate was dissolved in 100 mL of water, 10 g of sodium acetate was added, and 10 g of iodine was added dropwise (dissolved in 100 mL of dimethylformamide ), stirred and reacted at 50-70° C. for 12 hours. The reaction solution was diluted with water to 400mL, dynamically adsorbed with 100mL D151 resin, washed with water, and eluted with 0.4N ammonia water. When the pH was 9.5, it began to be collected in sections. The same components were combined and evaporated to dryness under reduced pressure. Add 50 mL of water to dissolve, purify with HD-2 (weakly acidic cationic resin) resin column (height / diameter > 10), elute with 0-0.4N ammonia water, and collect in sections. Combine the same components, concentrate to dryness, dissolve with 30mL water, add 6N H 2 SO 4 Adjust the pH to 4.5-7, add 1 gram of activated carbon ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com