Method for preparing atorvastatin calcium intermediate
A technology of atorvastatin calcium and system, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of long synthesis route, complicated operation, expensive raw materials and the like, and achieves the effects of less pollution, simple operation and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
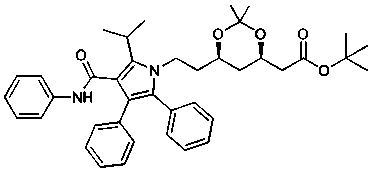
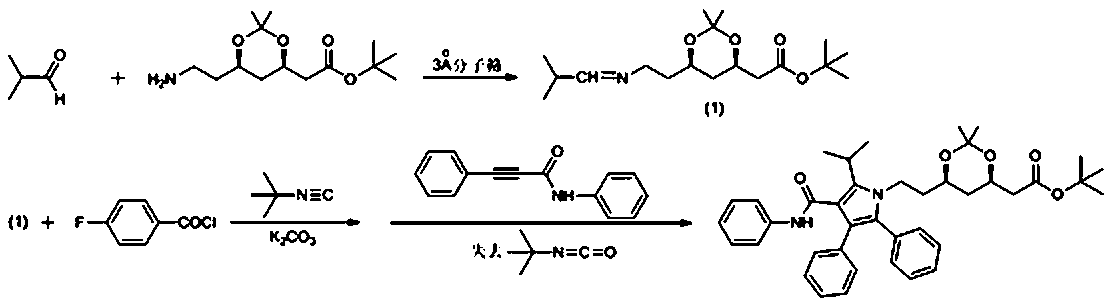
[0029] Under the protection of nitrogen, 1.73g isobutyraldehyde, (4R,6R)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane Alk-4-yl] tert-butyl acetate 5.46g, tetrahydrofuran 15ml, add 5g 3Å molecular sieve 100ml two-necked flask (with stirring magnet), stir at room temperature for 12-24h; under the protection of nitrogen, use a syringe to 2.34g of p-fluorobenzoyl chloride was slowly added dropwise to the reaction solution with constant stirring. After the addition, after reacting for 1h at room temperature, 1.66g of tert-butyl isonitrile was added dropwise to the reaction solution with a syringe and continuously stirred. After completion, after reacting at room temperature for 1 hour, 3.17g of potassium carbonate was added to the above reaction solution, and 4.42g of 3-phenylpropioanilide was added dropwise to the reaction solution with a syringe, and stirring was continued. After the addition, React at room temperature for 24 hours. After the reaction is complete, extract with ethyl ...
Embodiment 2
[0031] Under the protection of nitrogen, 1.73g isobutyraldehyde, (4R,6R)-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane Alk-4-yl] tert-butyl acetate 5.46g, tetrahydrofuran 15ml, put into a 100ml two-necked flask with 3Å molecular sieve 5g, magnet, stirred at room temperature for 12-24h; then the molecular sieve in the above reaction solution was filtered out, Under the protection of nitrogen, use a syringe to slowly add the filtrate dropwise to a 100ml two-necked flask (with stirring magnet) containing 3.17g of p-fluorobenzoyl chloride, 3.31g of potassium carbonate, and 10ml of tetrahydrofuran, and continue to stir. Then, after reacting for 1 hour at room temperature, 1.66 g of tert-butyl isonitrile was added dropwise to the reaction solution with a syringe and stirring continuously. After the addition, after reacting for 1 hour at room temperature, 3.31 g of potassium carbonate was added, and the 3- 4.42g of phenylpropionylanilide was added dropwise to the reaction solution and sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com