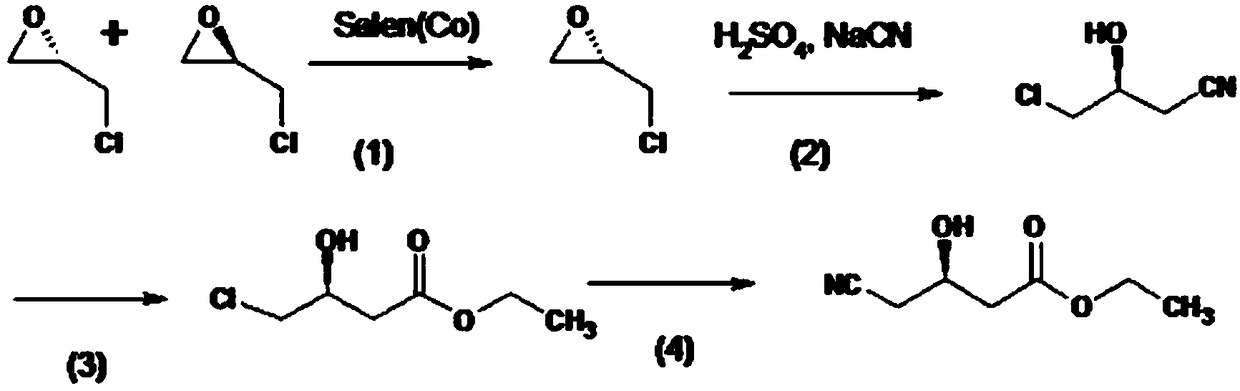
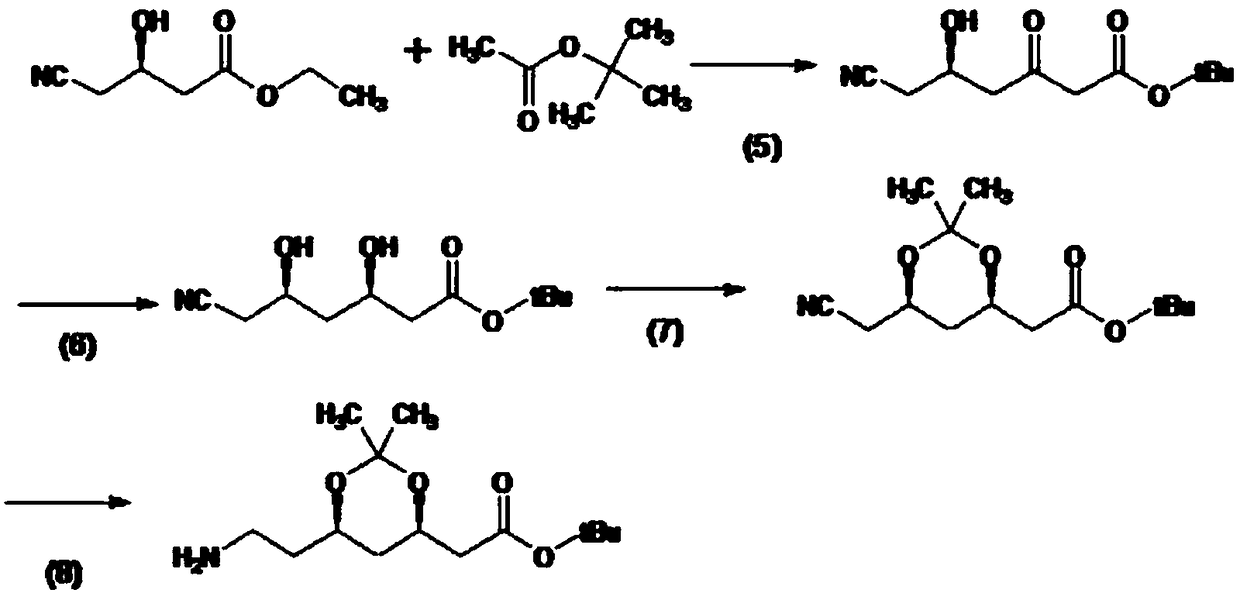
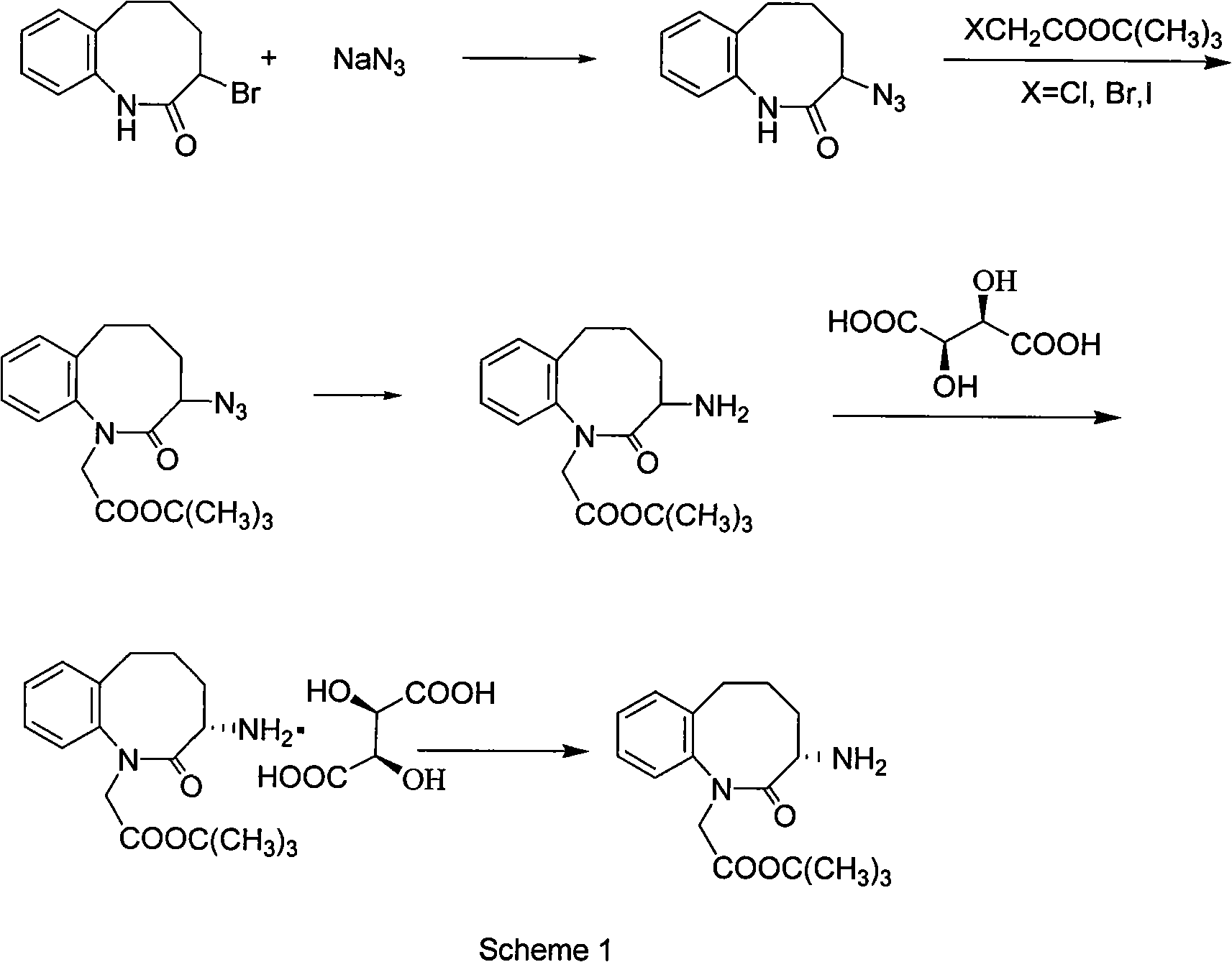
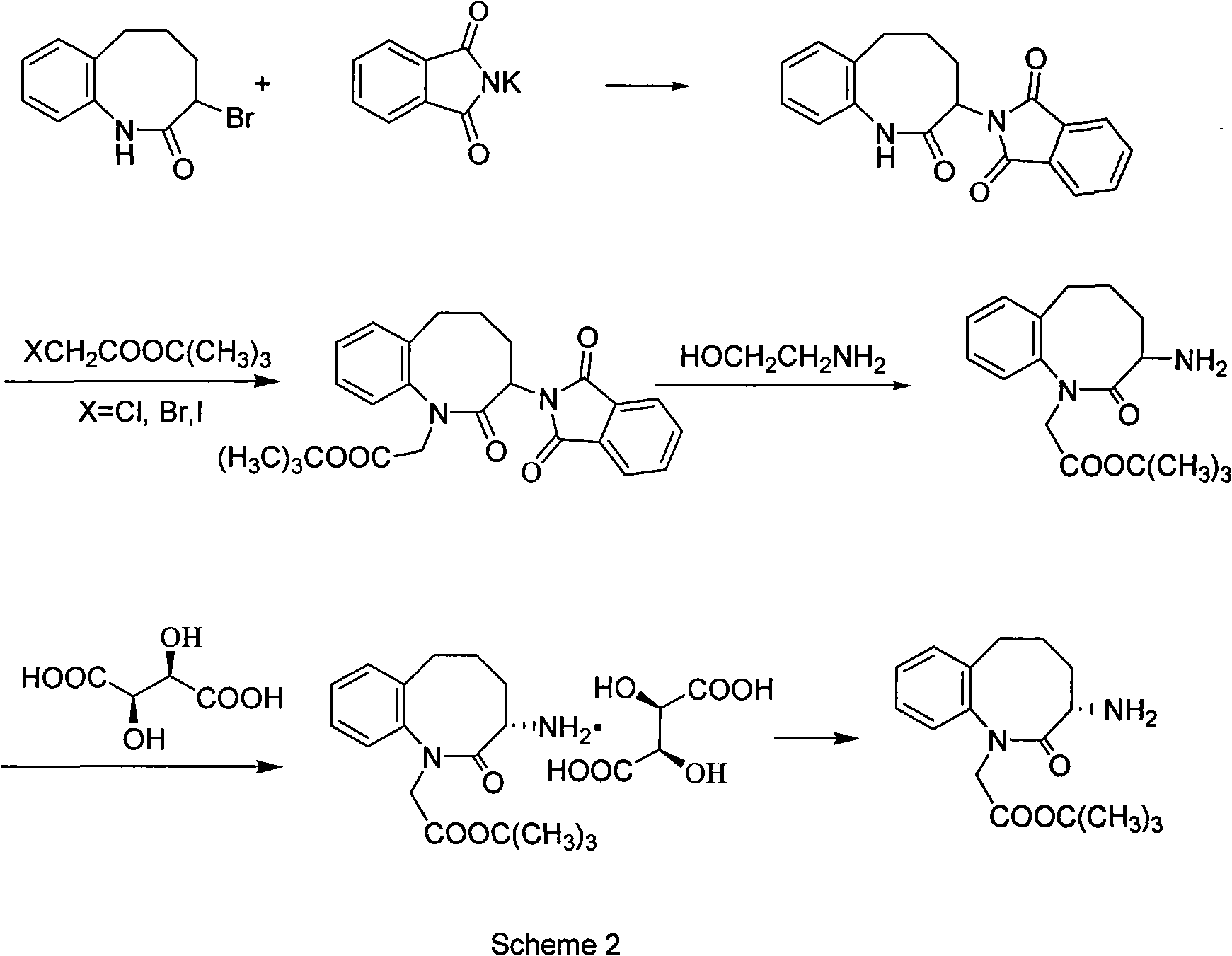
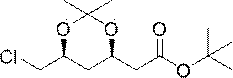Patents
Literature
62 results about "Tertiary butyl acetate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tert-Butyl acetate, t-butyl acetate or TBAc is a colorless flammable liquid with a camphor- or blueberry-like smell. It is used as a solvent in the production of lacquers, enamels, inks, adhesives, thinners and industrial cleaners.
Inkjet inks and methods of printing with the same
Described herein are inkjet inks that are substantially free of volatile organic compounds, or have a relatively small amount of volatile organic compounds, that are considered to significantly contribute to the formation of ground level ozone. The inkjet inks include at least one resin selected from the group consisting of a vinyl chloride-vinyl acetate copolymer resin and a copolyester resin; at least one colorant; and a solvent mixture that includes acetone and at least one additional solvent selected from the group consisting of tertiary butyl acetate and parachlorobenzotrifluoride. Also described are methods of printing indicia with inkjet inks.
Owner:GEM GRAVURE
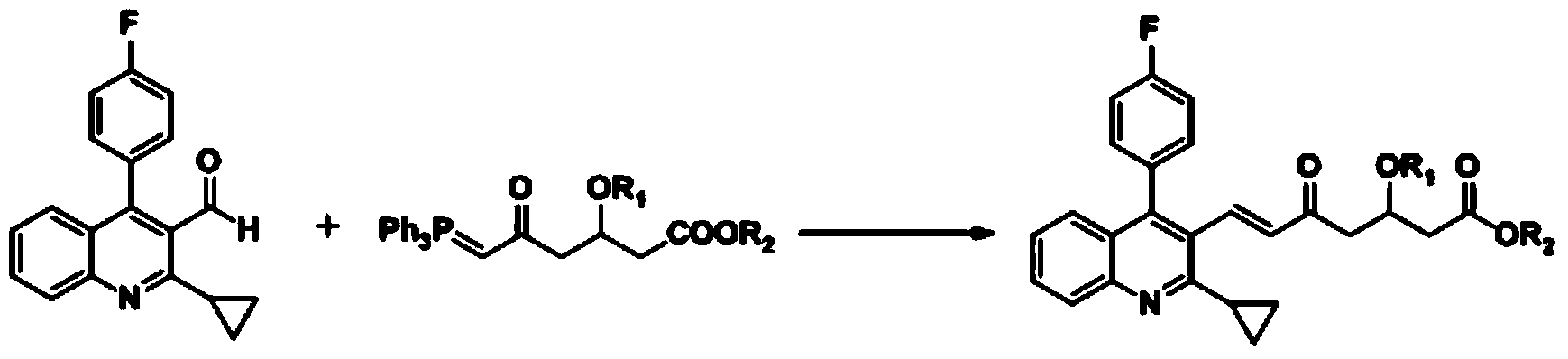
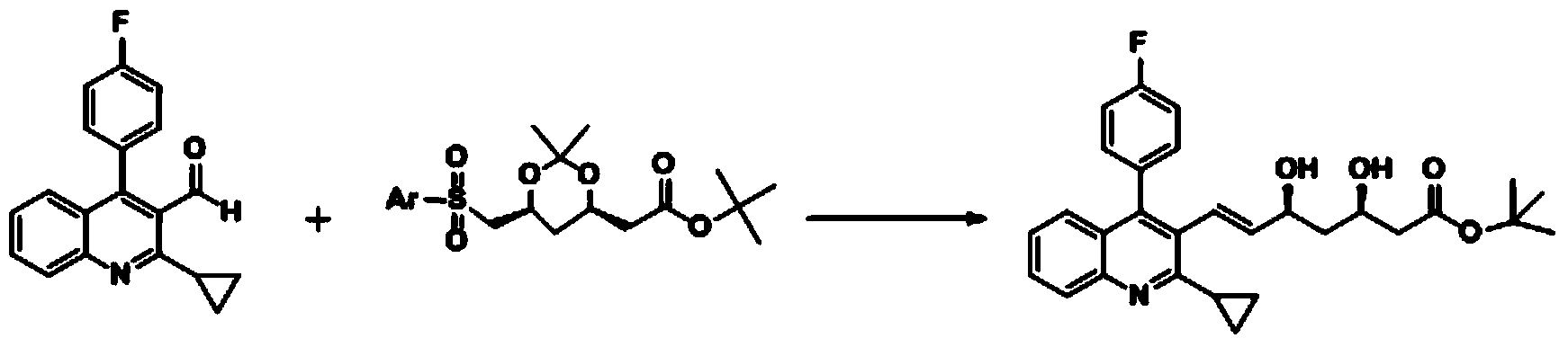
Method for preparing solid (4R-cis)-6-formyl-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate
The invention discloses a method for preparing solid (4R-cis)-6-formyl-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate and belongs to the pharmaceutical and chemical field. The method includes the following steps that a compound III is dissolved in a mixed solvent of methanol and water, potassium carbonate is added for hydrolysis, potassium carbonate is filtered out and removed, methanol is removed through distillation, an organic solvent is added, and an organic solvent solution of a compound II is obtained after washing; secondly, TEMPO, potassium bromide and sodium bicarbonate are added, stirred, mixed and cooled, then a sodium hypochlorite solution is added dropwise, stirring reaction is carried out, after reaction, a sodium thiosulfate solution is quenched, washed, dried and condensed, and a crude product of a compound I is obtained; thirdly, an organic solvent is added, cooled and stirred after being heated and dissolved and is crystallized and filtered to obtain solid crystal of the compound I. The preparation method is safe, environmentally friendly, easy and convenient to operate, high in yield, high in purify and capable of facilitating large-scale production.
Owner:VALIANT CO LTD
Reverse sex pheromone for prevention and control of plutella xylostella l.
InactiveCN105123718AInhibition orientationInterfering with matingBiocidePest attractantsPropionateMammal
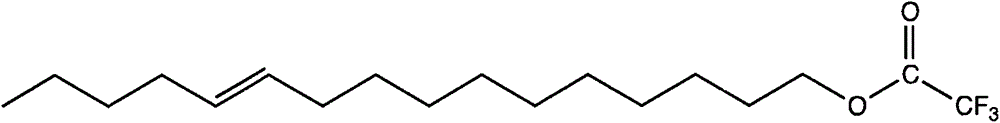
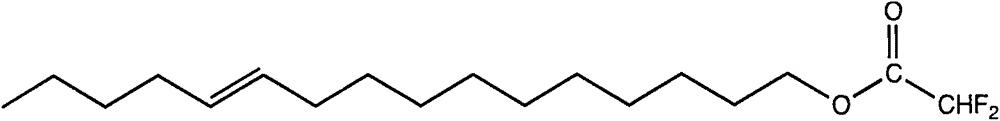
The invention discloses a reverse sex pheromone for interfering plutella xylostella l. mating, and belongs to the biological prevention and control field. The reverse sex pheromone is one or more of cis-11-cetylenol trifluoromethyl ketone, cis-11-cetylenol difluoromethyl ketone, cis-11-hexadecene-1-propionate and cis-11-hexadecene-1-tertiary butyl acetate. The reverse sex pheromone can significantly inhibit orientation of plutella xylostella l. male adults on female sex pheromone, thereby interfering plutella xylostella l. mating, and reducing the field insect population number. The reverse sex pheromone has the advantages of high efficiency, multiple ecological effects, simple preparation, environmental friendliness, safety to mammals and the like, can reduce the field male and female meeting probability and mating frequency, so as to relieve harm of plutella xylostella l. on cruciferous plants.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
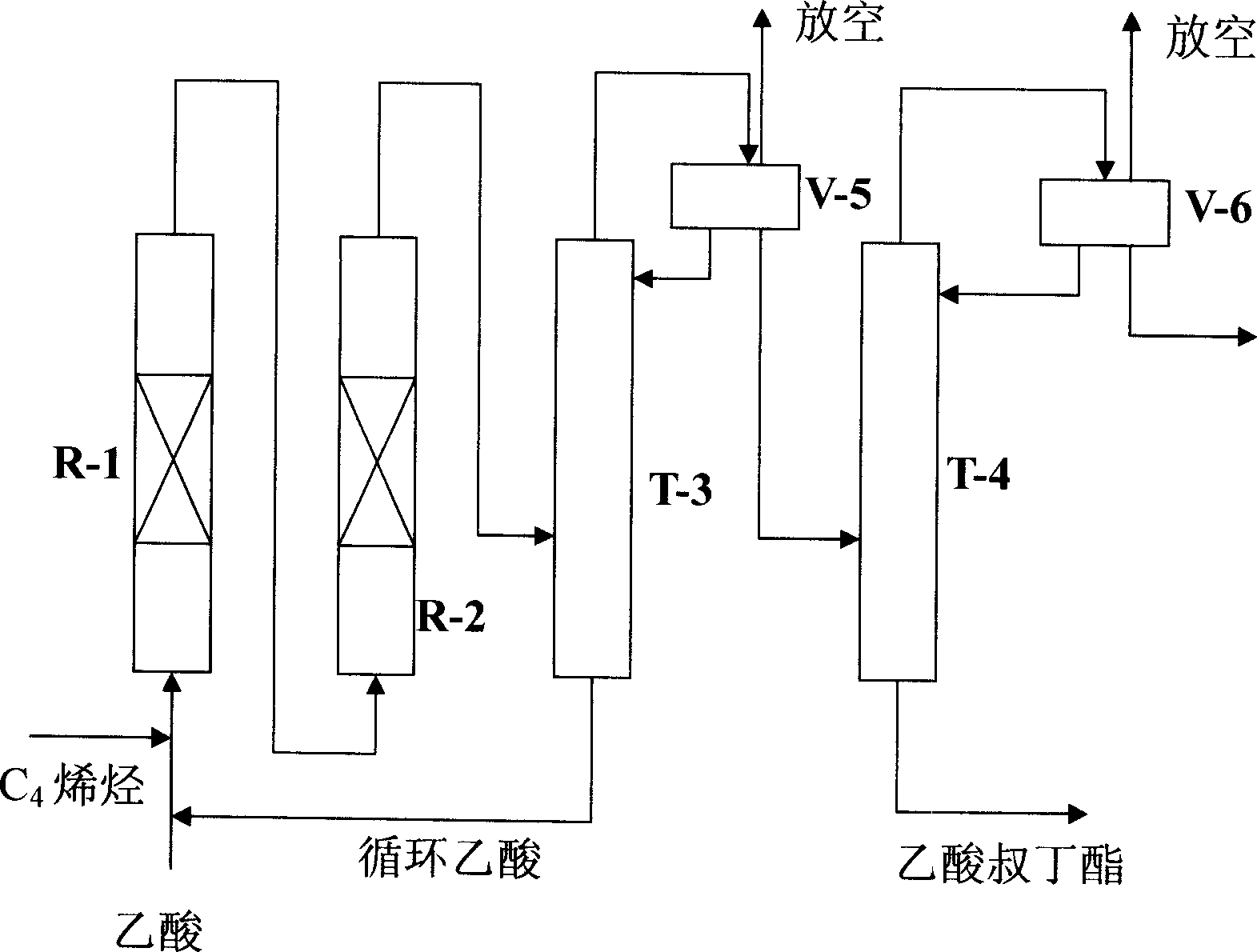
Process for preparing and extracting tert-butyl acetate
ActiveCN1884248AReduce manufacturing costOvercome increased energy consumptionOrganic compound preparationCarboxylic acid esters preparationAcetic acidAlcohol
The invention discloses a preparing and extracting method of tert-butyl acetate, which is characterized by the following: adding acetate and C4 with isobutene continuously at 1:0.30-1:5.00 molar rate in the esterification tower with strong acid ionic exchange resin catalyst; setting inlet air-speed of acetate at 0.5-5.0 h-1 and catalyst quantity at 30-90 ml and pressure at 0.5-2.0 Mpa at 30-110 deg.c; obtaining coarse ester; sending ester in the separating tower; controlling top-gas reflux temperature at 85-100 deg.c to deacidify; dehydrocarbylating at 70-85 deg.c; dealcoholizing at 78-90 deg.c; rectifying between 90 and 100 to obtain the product.
Owner:HUNAN CHANGLING PETROCHEM SCI & TECH DEV CO LTD
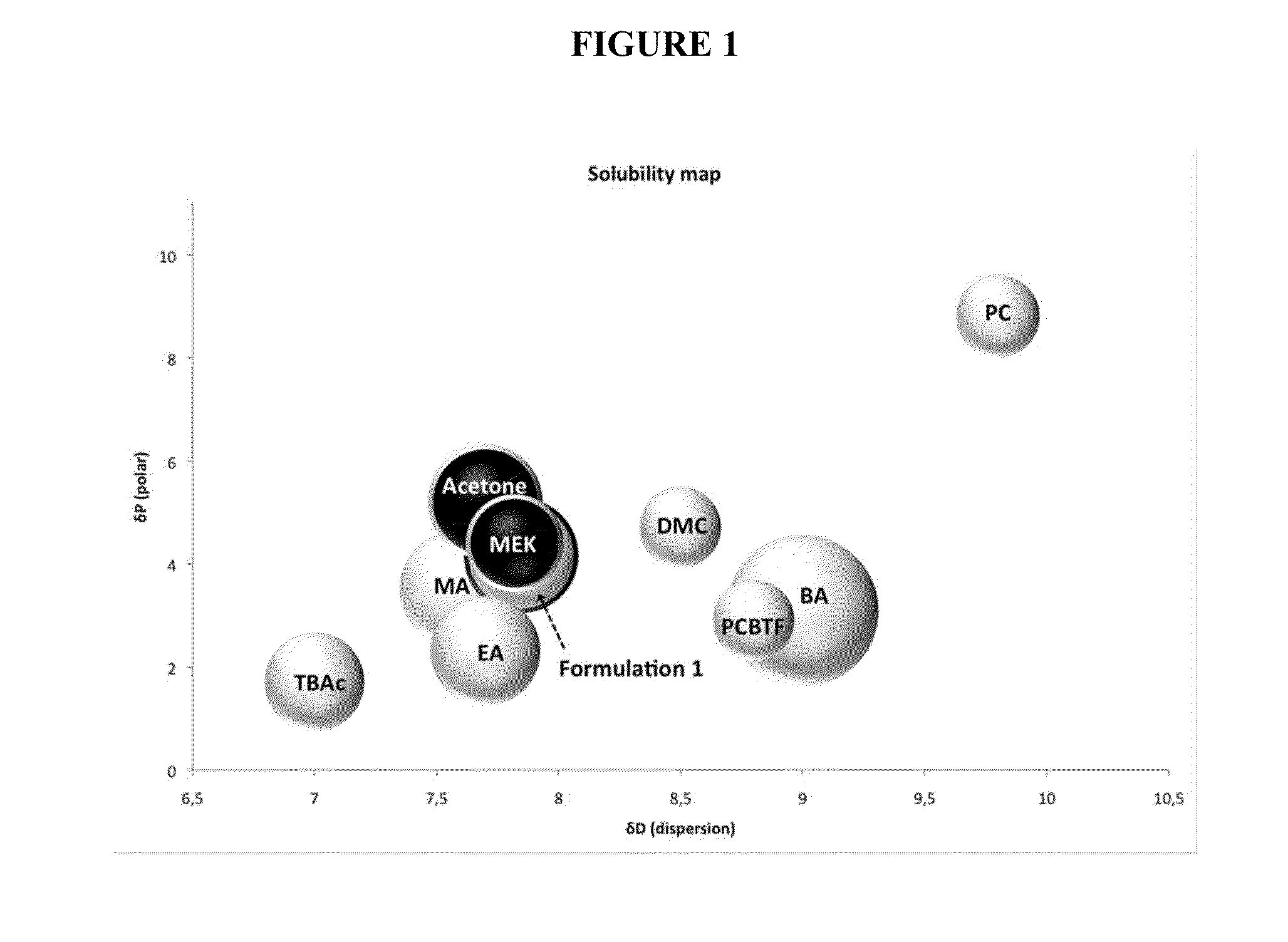
Solvent formulations
The present disclosure provides, in part, a solvent composition including an an acetic acid alkyl (C1-C4) ester (e.g., methyl acetate (MA), ethyl acetate (EA), or tert-butyl acetate (TBAc)) and a carbonate ester (e.g., dimethyl carbonate, or propylene carbonate).
Owner:TBF ENVIRONMENTAL TECH
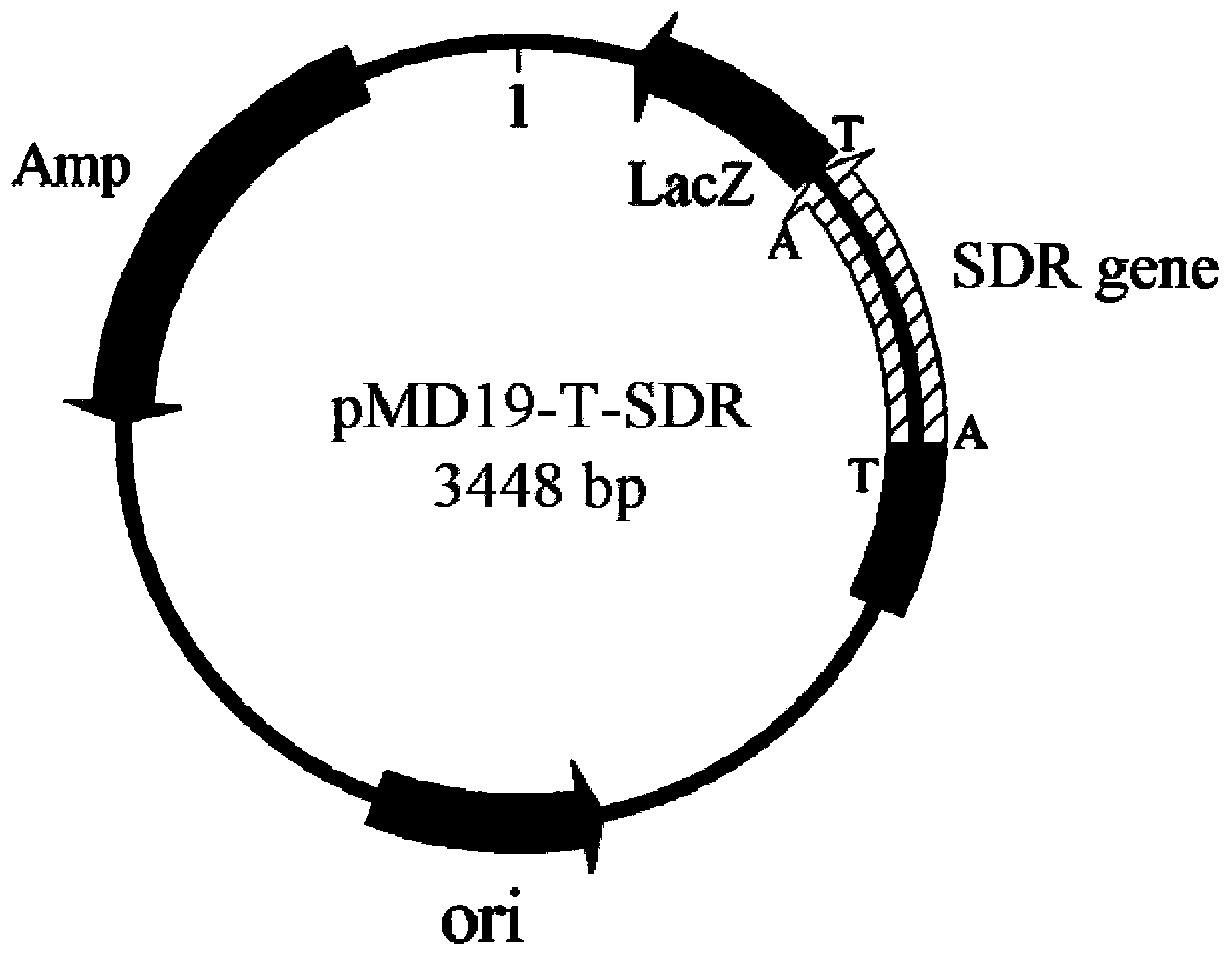
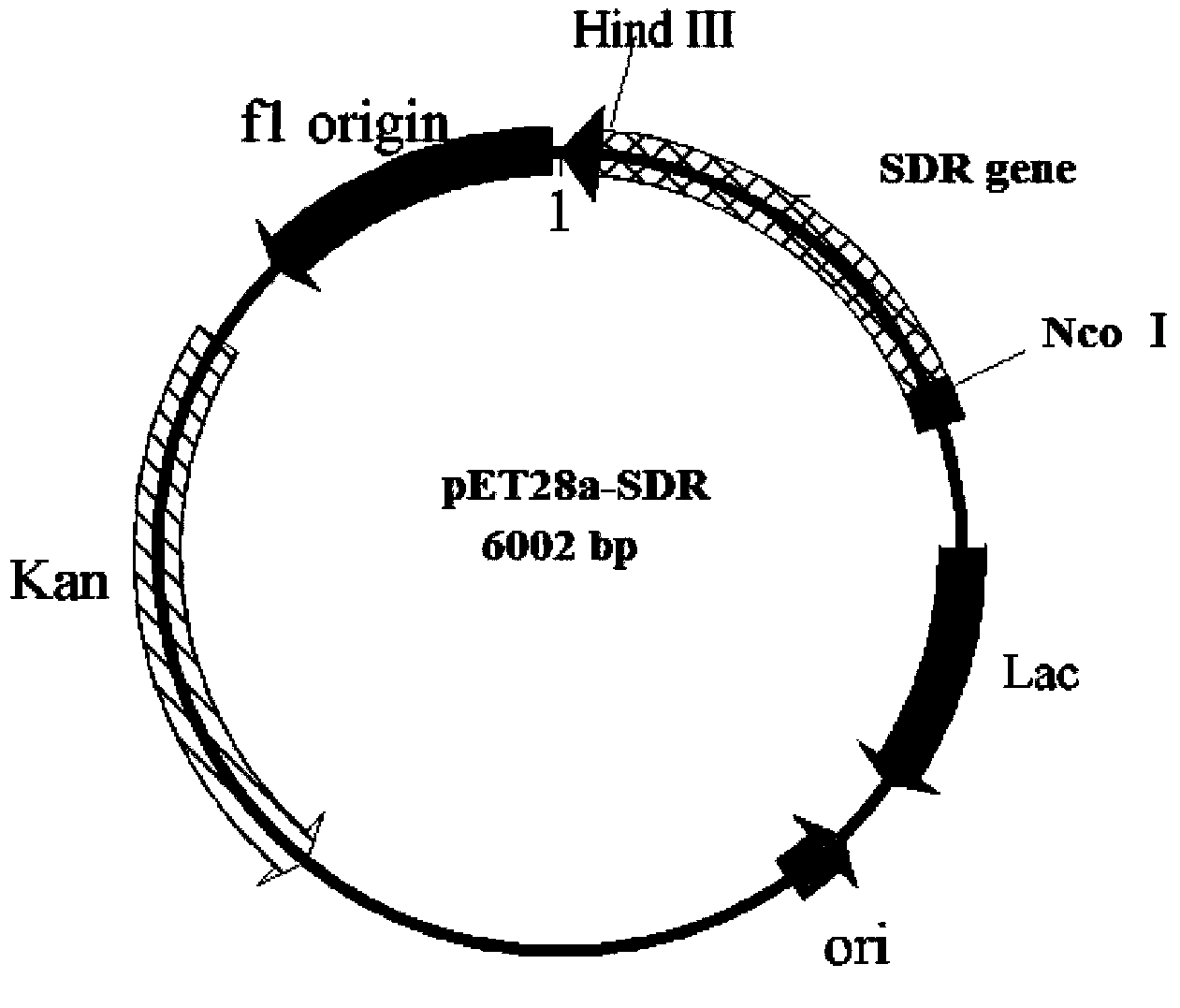
Leifsonia xyli HSO904-based short chain dehydrogenase, and encoding gene, carrier, engineering bacteria and application thereof
The invention discloses a leifsonia xyli HSO904-based short chain dehydrogenase, and an encoding gene, a carrier, engineering bacteria and application thereof. A gene of the leifsonia xyli HSO904-based short chain dehydrogenase has more than 90% of homology of a nucleotide sequence shown in SEQ ID NO. 1. A colon bacillus BL21 / pET28a (+)-SDR prepared by the recombination of the short chain dehydrogenase is used as an enzyme source, 3, 5-bis-trifluoro methyl acetophenone, trifluoromethyl acetophenone, 4-hydroxyl-2-butanone, acetoacetic ester, 4-chloro ethyl acetoacetate, acetoacetic acid tert-butyl acetate and the like are used as substrates to prepare corresponding chiral compounds such as (R)-3, 5-bis-trifluoromethyl phenethyl alcohol, trifluoromethyl benzaldehyde ethanol, 2-hydroxyl-butyl alcohol, 3-hydroxy ethyl butyrate, 4-chloro-3-hydroxy ethyl butyrate and 3-hydroxy butyric acid tert-butyl acetate through a catalytic asymmetric reduction reaction.
Owner:艾吉泰康(嘉兴)生物科技有限公司
Process for producing important synthesis midbody of high purity atorvastatin
ActiveCN101429195AEasy to recycleImprove conversion rateOrganic chemistryFiltrationTertiary butyl acetate
The invention relates to a method for preparing high-purity (4R, 6R)-6-{2-[5-isopropyl-3-phenyl-2(4-fluorophenyl)-4-(phenylcarbamoyl)-yrrol-1-yl]- ethyl}-2, 2-dimethyl-[1, 3]-dioxane-4-yl-tert-butyl acetate, which comprises the following steps: step one, [5-methyl-4-isopropyl-2-phenyl-1(4-fluorophenyl)-3-(phenylcarbamoy1)-1,4-hexanedione](II) and [(4R,6R)-2,2-dimethyl-6-(2-aminoethyl)-[1,3]-dioxane-4-yl-tert-butyl acetate](III) with a mol ratio of between 0.71 and 1.12 to 1 are weighed, an acid catalyst which is 1.05 to 1.15 times of mol number of the formula (II) is weighed, the mixture is dissolved in a non-hydroxy solvent which is 3.0 to 4.2 times of the weight of the formula (II) under the protection of nitrogen and the stirring, and heating reflux and azeotropic water entrainment are performed until an HPLC shows that the reaction is finished; and second two, the solvent is removed under vacuum, then a water-isopropanol mixed solvent with a volume ratio of 2 to 5 is used to recrystallize the mixture, and a key intermediate of synthetic atorvastatin calcium which has an HPLC purity not less than 99.0 percent and is expressed by the formula (I) is obtained after the pump filtration and drying. The method has the advantages of simple process, low equipment requirement, low cost, convenient and quick recovery of the solvent, less environmental pollution, and high product purity.
Owner:安徽美诺华药物化学有限公司
Lacquer thinner
A lacquer or other coating thinner having a low volatile organic compound (VOC) rating which permits its use for cleaning and thinning in government regulated areas. The thinner has an acetone, methyl acetate or tertiary butyl acetate or mixture as a base. It has various non-hazardous ingredients which include a soy oil material, a dibasic ester and a glycol and carbonate ingredient such as tetrahydrofurfuryl alcohol.
Owner:BORTZ DISTRIBUTING CO
3-site substituted (1-iso-indoxoline-2-base)piperidine-2,6-thiazolidinedione and synthetic method thereof
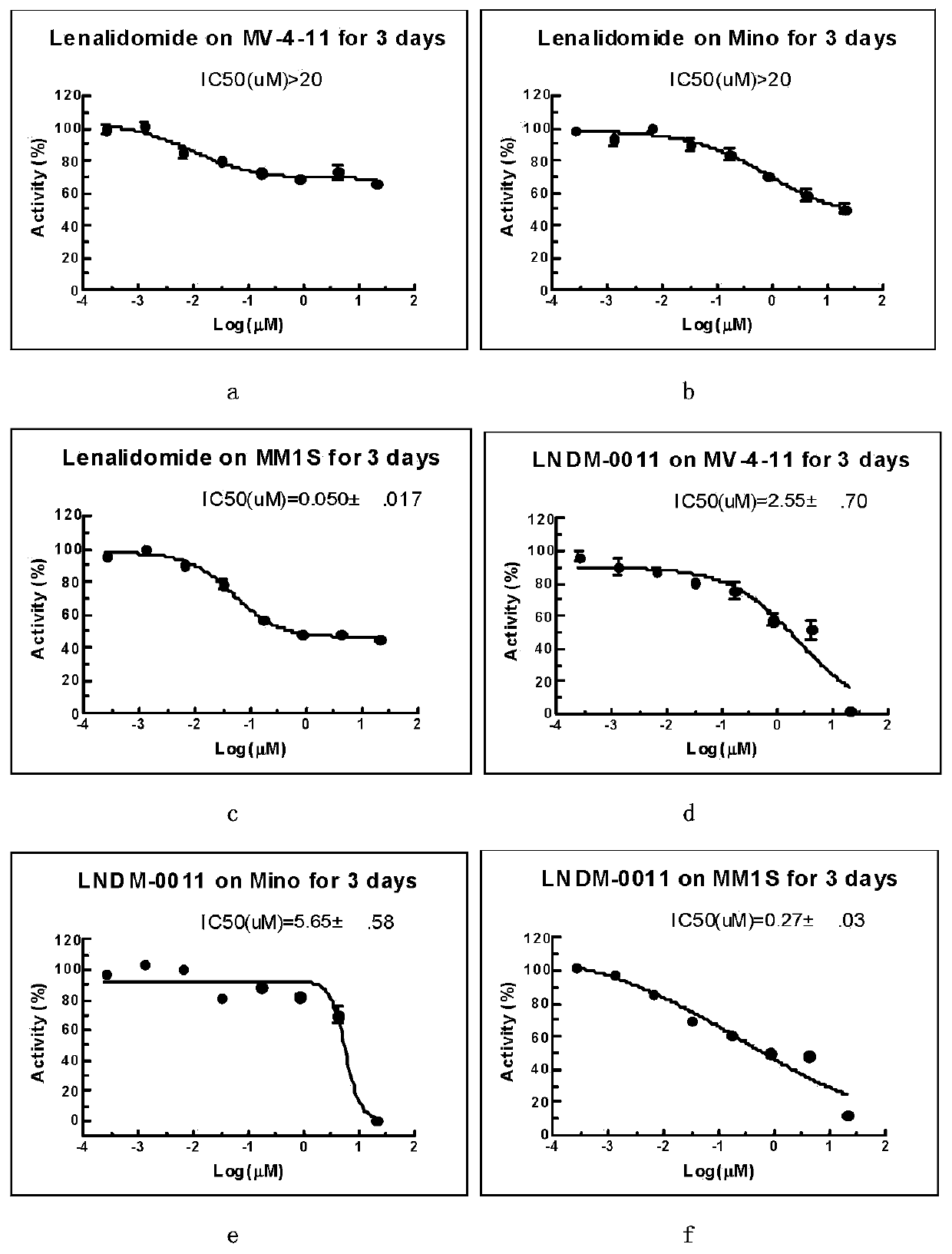
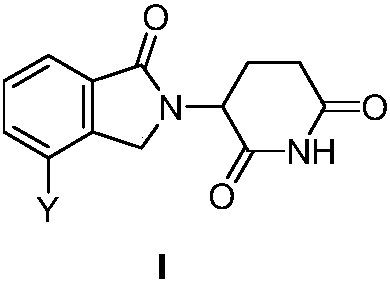
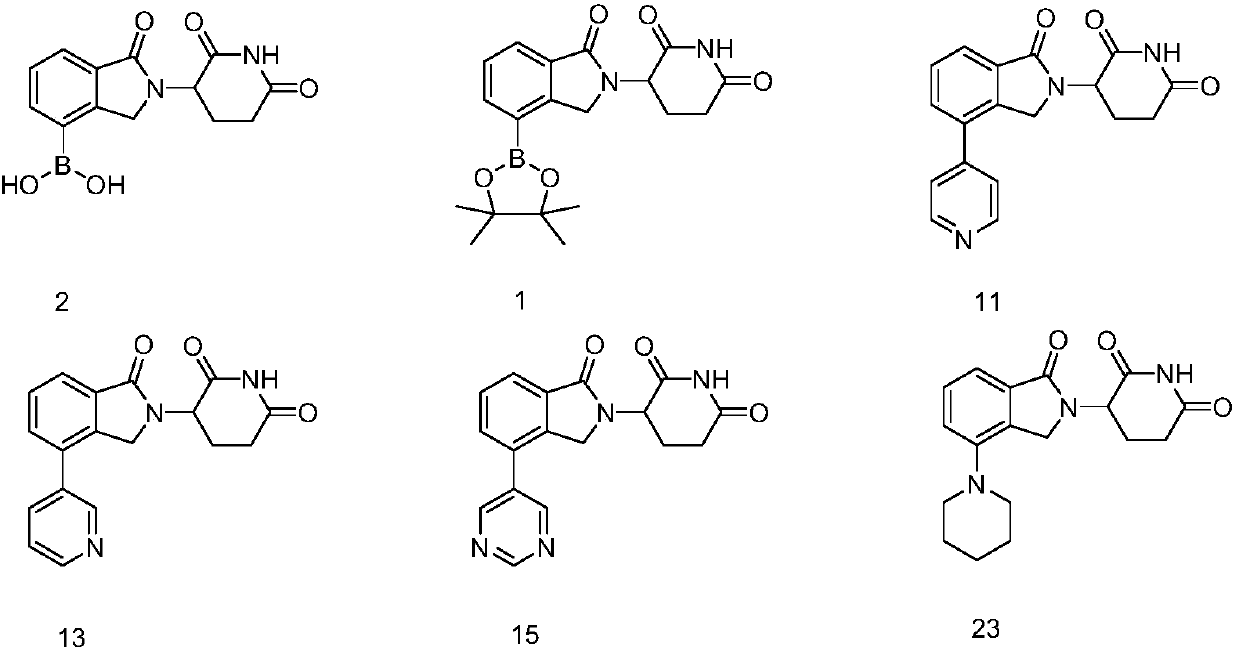
ActiveCN107739389AGroup 3/13 element organic compoundsAntineoplastic agentsMultiple myelomaTertiary butyl acetate
The invention discloses a 3-site substituted (1-iso-indoxoline-2-base)piperidine-2,6-thiazolidinedione and a synthetic method thereof, and belongs to the technical field of medicine synthesis. In a formula I, Y can be Z, R1, R2, -(CH2)n-R3, wherein Z is a boric acid ester group or a boric acid group, R1 is hydroxyl, cyanogroup or trifluoromethyl, R2 is morpholinyl, piperidyl and methyl piperazinegroup; in the formula -(CH2)n-R3, R3 is alkylene, tert-butyl acetate, phenyl group and heterocyclic aromatic group, and n is 0 or 1. The compound can be used for preparing medicines capable of treating or preventing multiple myeloma, leukemia and lymphoma. The formula is shown in the description.
Owner:EAST CHINA NORMAL UNIVERSITY
Preparation process of tert-butylhydroquinone
ActiveCN105294403AReduce usageLow DTBHQ contentOrganic chemistryOrganic compound preparationWarm waterEthylic acid
The invention relates to the technical field of antioxidant preparation methods, and particularly relates to a preparation process of tert-butylhydroquinone (TBHQ). The preparation process comprises the following steps of (1) simultaneously adding excessive dilute sulphuric acid, equimolar hydroquinone and tertiary butyl acetate to a reaction still, wherein the weight ratio of the dilute sulphuric acid to the tertiary butyl acetate is 1:(3 to 6); (2) stirring and heating to 85 to 95 DEG C, and reacting for 1 to 8 hours; (3) stopping stirring, cooling to 65 to 75 DEG C, and then performing centrifugal filtration, wherein filtrate of sulphuric acid is recycled, and obtained filter residues are washed with 1 to 3 times warm water, so as to obtain a crude product of the TBHQ. Compared with the prior art, the preparation process has the advantages that the hydroquinone and the tertiary butyl acetate are used as raw materials, and the dilute sulphuric acid is used as a catalyst and a reaction solvent, so as to promote a reaction; tertiary butyl is supplied by the tertiary butyl acetate, so that side reactions are reduced; the crude product contains 70% to 75% of TBHQ, the content of DTBHQ (Di-Tert-Butylhydroquinone) as a by-product is low, the yield can reach 65 to 70% after purification, the yield is greatly increased, and great economic benefits are obtained.
Owner:东莞市感恩食品科技有限公司
Environment friendly energy conservation fuel and preparation thereof
InactiveCN101319157AImprove combustion effectReduce dosageGaseous fuelsTertiary butyl acetateProcess engineering
The present invention relates to an environment-friendly energy saving fuel, which is characterized in that the environment-friendly energy saving fuel comprises dimethyl ether. Further, the environment-friendly energy saving fuel also comprises an energy saving synergist. The compositions in portion by weight of the synergist are: 10 to 20 portions of methyl tert-butyl ether; 5 to 15 portions of tertiary butyl acetate; 10 to 20 portions of acetone; 10 to 20 portions of cyclopentanone; 5 to 15 portions of xylene; 10 to 20 portions of petroleum ether; and, 5 to 15 portions of ethanol. The environment-friendly energy saving fuel improves the combustion performance of the fuel with the flame temperature being high. The fuel reaches the same combustion efficiency with the consumption amount of the fuel gas being saved by between 20 and 50 percent. Additionally, the price of dimethyl ether is relatively cheap. Specifically, the price of dimethyl ether: the price of the rest burning gas is 5:6 to 7, i.e., people can save the cost of fuel by largely using dimethyl ether as fuel or part of fuel. The reduction of the consumption amount of the rest burning gases facilitates the reduction of the side effect of the burning gases. The present invention also relates to a preparation method of the environment-friendly energy saving fuel.
Owner:堆龙德庆金晋企业管理有限公司
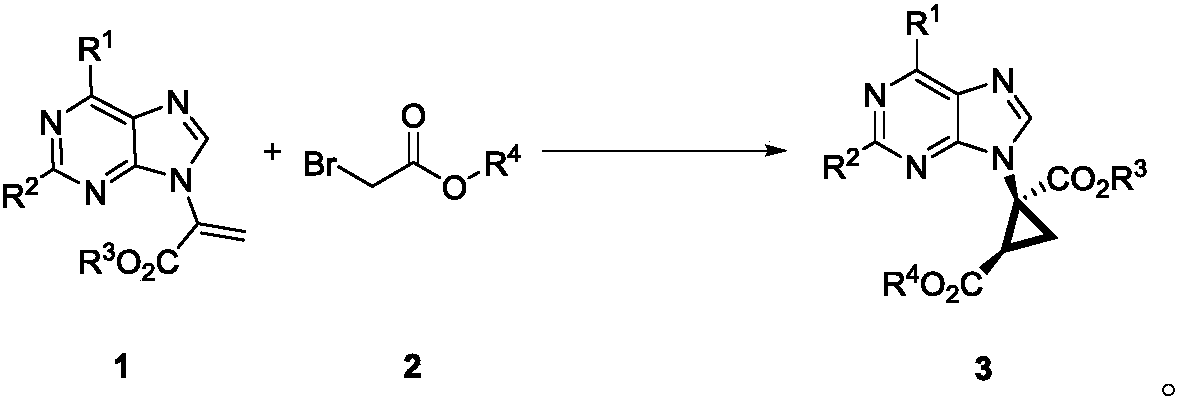
Method for synthesizing chiral ternary carbocyclic nucleoside through asymmetric cyclopropanation triggered by Michael addition
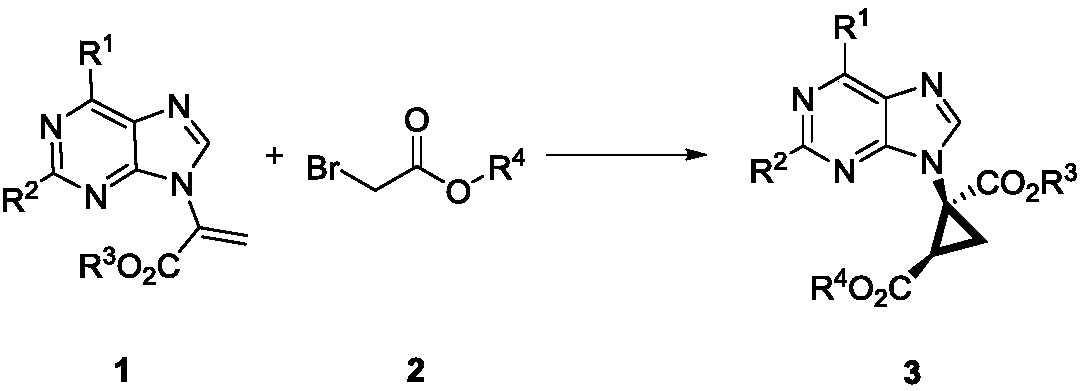
The invention discloses a method for synthesizing chiral ternary carbocyclic nucleoside through asymmetric cyclopropanation triggered by Michael addition and belongs to the field of asymmetric synthesis in organic chemistry. Chiral cyclopropane carbocycle purine nucleoside is prepared from alpha-purine substituted acrylate and bromo-tert-butyl acetate as raw materials after a chiral amine catalysis reaction derived by quinine, the reaction enantioselectivity is good, and the yield is medium to excellent.
Owner:HENAN NORMAL UNIV
Process for separating tert-butyl acetate in medicine waste solvent through vacuum rectification by using super-gravity bed
InactiveCN107827750AReduce manufacturing costImprove recycling ratesOrganic compound preparationVacuum distillation separationAcetic acidReflux
The invention relates to a vacuum rectification method using a super-gravity bed as core separating equipment, and discloses a process for separating and recycling a waste solvent in the synthetic process of statins or other medicines and recovering tert-butyl acetate in the solvent. During operation, the waste solvent enters from a place located between the two-layer and three-layer rotors of thesuper-gravity bed, wherein the rotating speed is 1000r / min, the reflux ratio is 4-10, the vacuum degree at the top of a tower is 20kPa-70kPa, and the temperature of a tower kettle is controlled at 70DEG C-85 DEG C, a tert-butyl acetate product with higher purity can be recovered in the tower kettle, the purity can reach 95% or more, the residual amount of isoprene is less than 1%, and the moisture content is less than 1000ppm, thereby fully meeting the requirements of recycling; and compared with traditional tower-type rectification operation, the process provided by the invention has the advantages of having a simple technological procedure, low energy consumption, a small occupied area of equipment, and high recovery rate of the product tert-butyl acetate, and being capable of effectively improving economic benefits.
Owner:南京揽博环境技术有限公司 +1
Method for preparing pitavastatin calcium
InactiveCN103508948ASolve the technical bottleneck of synthesisAchieve separationOrganic chemistryAcetic acidAfter treatment
The invention discloses a method for preparing pitavastatin calcium. The method comprises the following step: performing seven-step synthesis on 3-cyclopropyl-oxopropionate (comprising methyl ester and ethyl ester), (2-aminophenyl)(4-fluorophenyl) ketone and (4R-cis)-6-[(acetoxy)methyl]2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate which are used as initial raw materials so as to obtain the product. The reaction in each step is a conventional reaction and is suitable for large-scale production. The process is stable, the reaction conditions are mild, the after-treatment operation is simple, the intermediate is easy to separate, and the technical bottleneck in the existing pitavastatin calcium synthesis is solved.
Owner:ASYMCHEM LAB TIANJIN +4
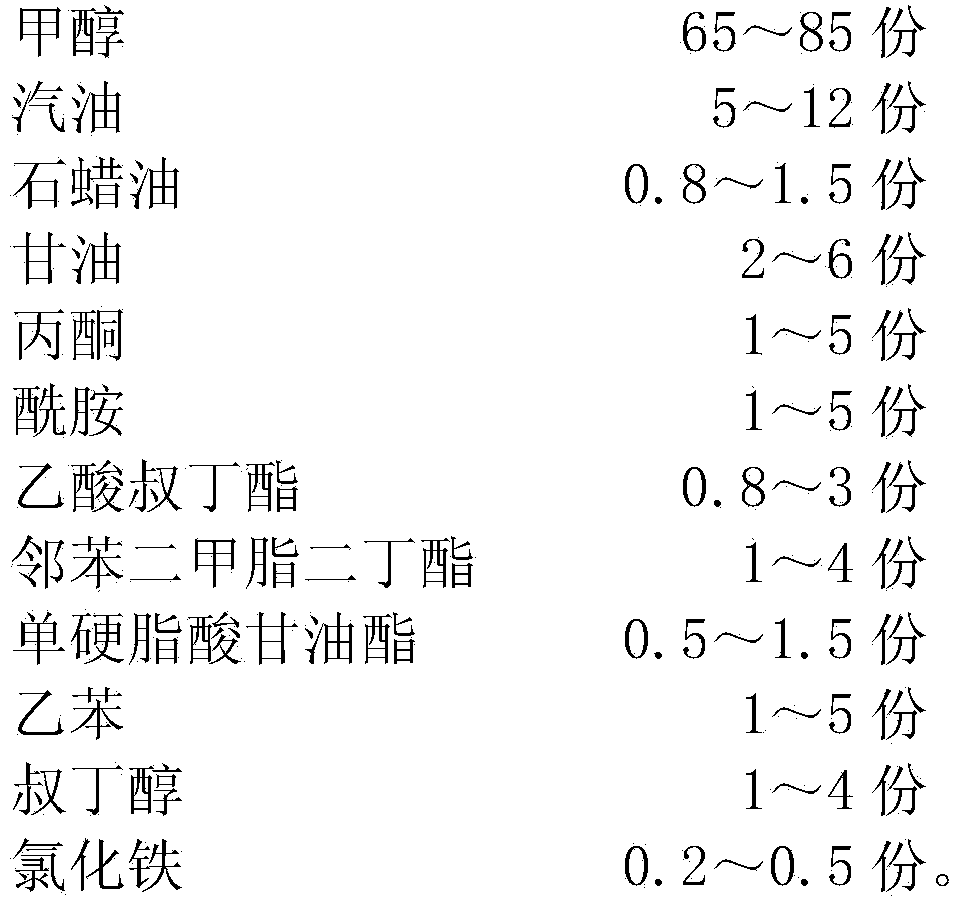
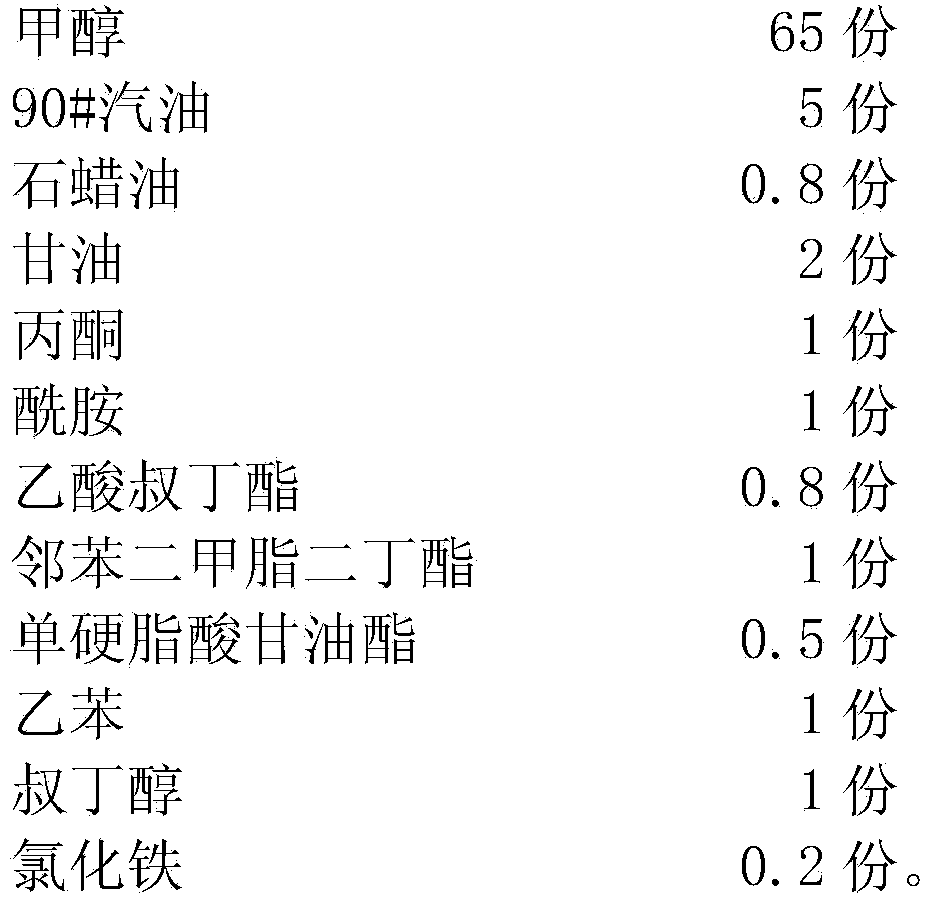
Methanol petrol for vehicles
InactiveCN104263424AHigh calorific valueStable combustionLiquid carbonaceous fuelsCarbon depositGlyceryl monostearate
The invention discloses methanol petrol for vehicles, and belongs to the field of fuel. The methanol petrol is prepared from the following raw materials in parts by weight: 65-85 parts of methanol, 5-12 parts of petrol, 0.8-1.5 parts of paraffin oil, 2-6 parts of glycerine, 1-5 parts of acetone, 1-5 parts of acidamide, 0.8-3 parts of tertiary butyl acetate, 1-4 parts of dimethyl phthalate dibutyl, 0.5-1.5 parts of glyceryl monostearate, 1-5 parts of ethybenzene, 1-4 parts of tert-butyl alcohol, and 0.2-0.5 part of iron chloride. The oxygen content of the methanol in the methanol petrol for the vehicles is high, the methanol petrol is sufficiently combusted, black smoke is not generated, carbon deposits are not generated, the bottom of a pan is not sooted, raffinate and residues are not generated, the exhaust emission amount after the combustion of the methanol petrol is low, the methanol petrol is clean and environment-friendly, the product performance is stable, the calorific value is high, the cost is low, the methanol petrol is safe and reliable, an engine does not need to be modified, the methanol petrol can directly replace petrol, and the methanol petrol has a broad application scope.
Owner:东奇能源技术有限公司
Low VOC construction primer
ActiveUS20150275045A1Improve adhesionGroup 4/14 element organic compoundsPretreated surfacesAcetic acidSilanes
The present disclosure relates to a primer composition. The primer composition comprises: a tert butyl acetate solvent; an organometallic reagent selected from organotitanates, organozirconates, aluminum organometallic compounds, and any combination thereof; an organotin compound; a silane with at least 3 hydrolyzable groups; and a polyorganosiloxane resin. Optionally, the primer composition further comprises a second solvent different from the tert butyl acetate solvent.
Owner:DOW TORAY CO LTD +1
Water-resistant composite additive for vehicular fuel oil
InactiveCN104164260ADestruction of oxidation conditionsDestroy anti-oxidant and anti-corrosionLiquid carbonaceous fuelsImidazolidineTert butyl phenol
The invention provides a water-resistant composite additive for vehicular fuel oil. The water-resistant composite additive is prepared from the following components in percentage by weight: 40-70% of mutual solvent, 20-40% of oxidization-corrosion inhibitor, 2-6% of bactericide and 8-14% of auxiliaries; the mutual solvent comprises the following components in percentage by weight: 10-30% of methyl tert-butyl ether, 10-15% of tert-butyl acetate, 15-20% of dimethyl carbonate, 40-50% of a mixture of C3-C6 higher alcohols, and 5% of N-ethylpyrrolidine; the oxidization-corrosion inhibitor comprises the following components in percentage by weight: 30-50% of 2,6-tert-butyl phenol, 10-15% of Bis(1-methylpropyl)-1,4-phenylenediamine, 20-30% of T1201, 15-20% of methyl benzotriazole and 5% of a mixture of ethoxylated alkylphenol and oil-soluble imidazolidine according to the weight ratio of 6:4; the bactericide comprises the following components in percentage by weight: 40-60% of dioxolakylborane-containing solution, 15-30% of N-benzylideneaniline, 10-15% of polyethylene glycol borate, and 15% of 2-methyl-4-Isothiazolin-3-one; and the auxiliaries comprise the following components toluene, aviation kerosene, dioctyl sebacate and cyclohexane according to the weight ratio of 3:2:1:4. The water-resistant composite additive can effectively solve the problems that the vehicular fuel oil causes phase separation, serious corrosion and wear to an engine, and the like when absorbing water during production and storage and transportation.
Owner:ENERGY & ENVIRONMENT RES INST OF HEILONGJIANG PROVINCE
Preparation method of cis-rosuvastatin calcium impurity
ActiveCN105017158AQuality improvementOriginalityOrganic chemistryAfter treatmentTertiary butyl acetate
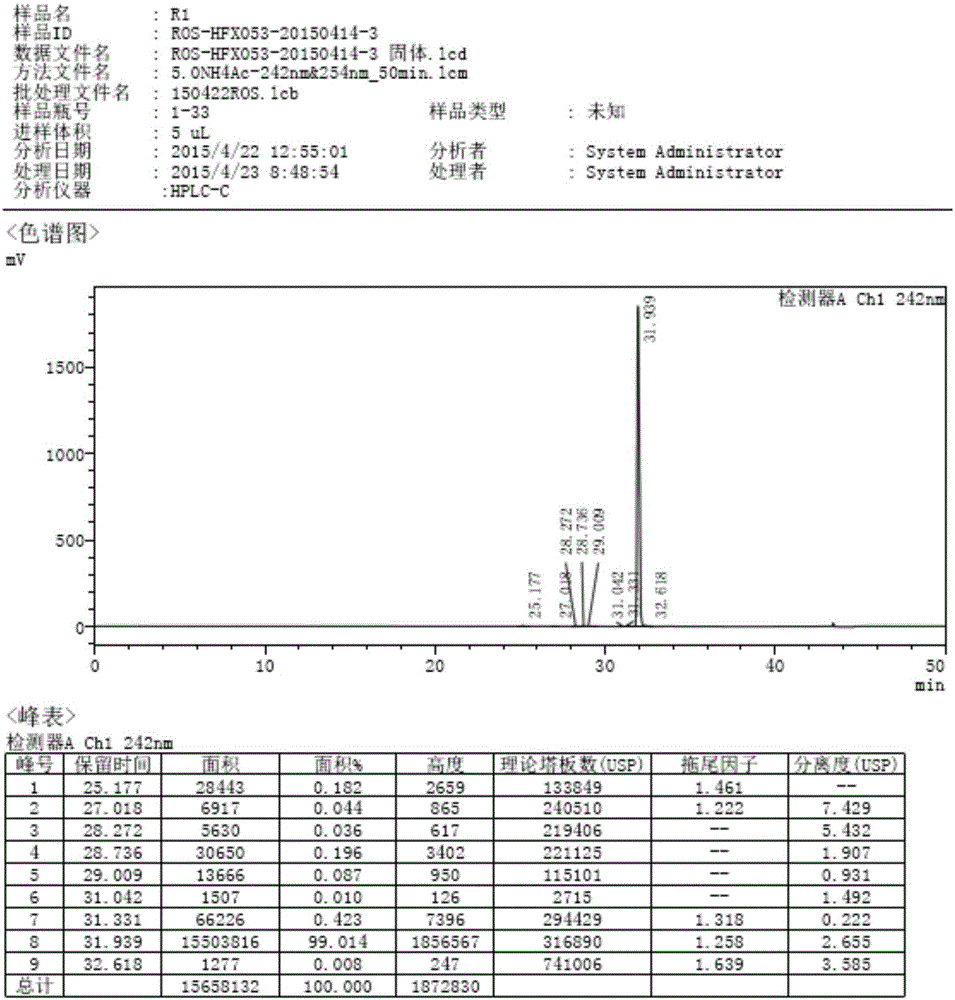
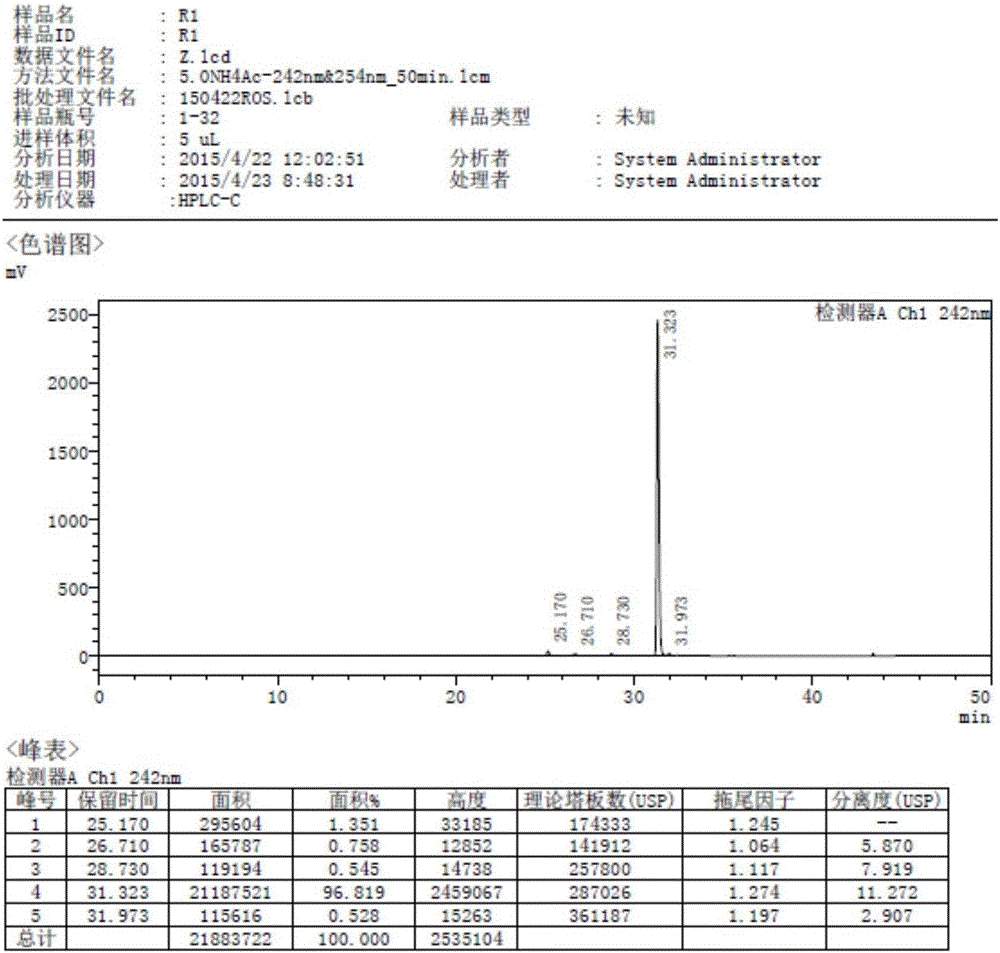
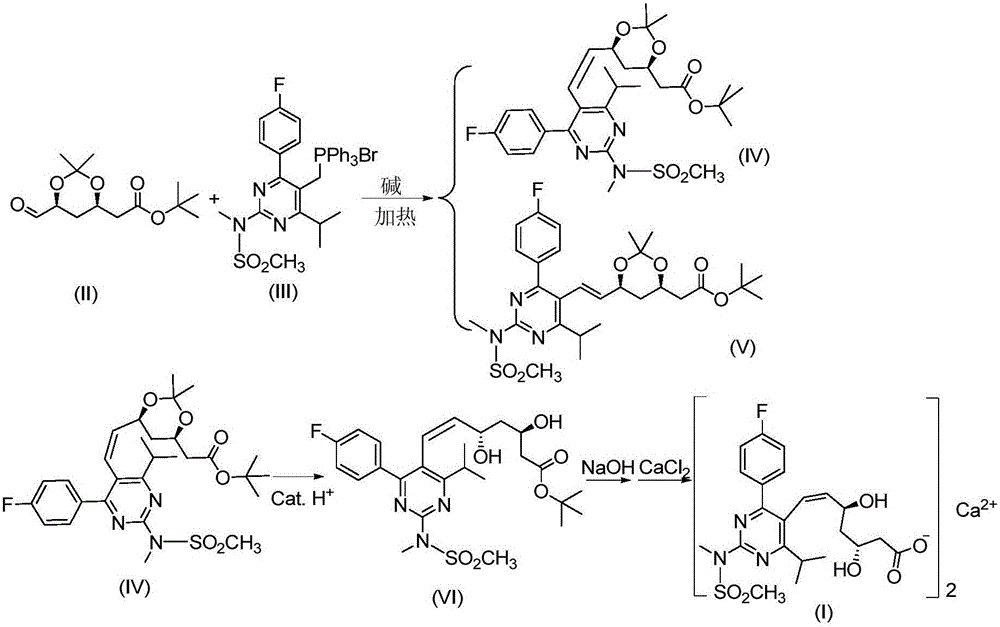
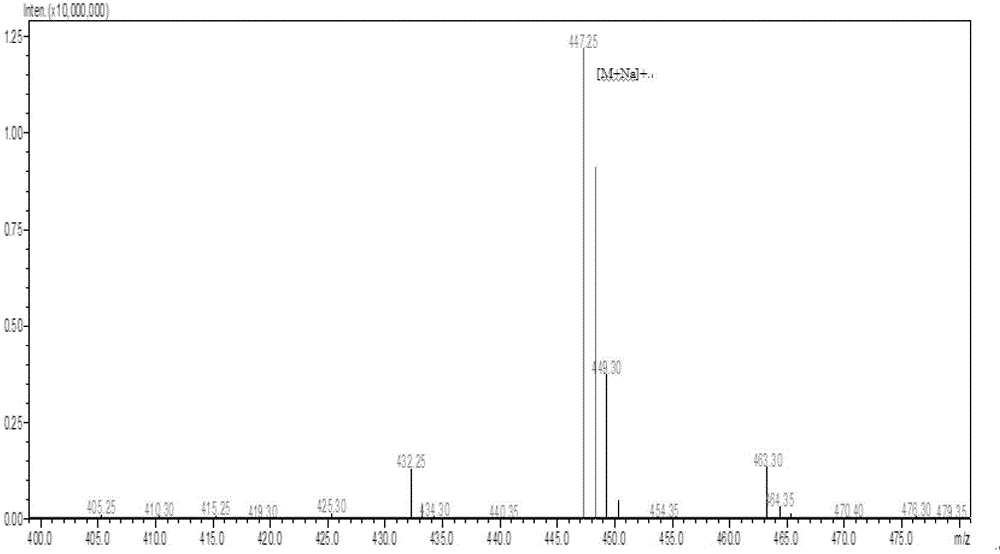
The invention discloses a preparation method of a cis-rosuvastatin calcium impurity. According to the preparation method, (4R-cis)-6-carbaldehyde-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate and [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)-5-pyrimidinyl]triphenylphosphonium bromide are used as the raw materials for preparing a target compound by virtue of Witting reaction, isomer separation, deprotection, hydrolysis and salt forming reaction. The method has the characteristics of originality, simplicity in operation, convenience in after-treatment, high product purity and yield and the like. The preparation method has the beneficial effect that certain help is provided for the registration, the declaration, the production and the center control of cis-rosuvastatin calcium as well as the quality improvement of cis-rosuvastatin calcium.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Environment-friendly wave-absorbing coating and preparation method thereof
ActiveCN109627832AEasy to useExtended service lifePolyurea/polyurethane coatingsRadiation-absorbing paintsBenzeneMethyl carbonate
The invention discloses an environment-friendly wave-absorbing coating and a preparation method thereof, and belongs to the field of wave-absorbing materials. The environment-friendly wave-absorbing coating comprises 7-73% of matrix resin, 9-63% of a wave-absorbing agent, 4-26% of a solvent, 0.5-2% of a dispersing agent, 0.4-1% of an anti-settling agent and 1-12% of a curing agent; wherein the solvent is selected from at least one of dimethyl carbonate, tert-butyl acetate, dimethyl adipate, dimethyl glutarate, dimethyl succinate, propylene glycol diacetate and propylene glycol methyl ether acetate. The environment-friendly wave-absorbing coating does not contain benzene, ketone and other solvents with high toxicity, high harm and carcinogenic risk, and has the advantages of environmental friendliness, low toxicity, low harm and no pollution; and a cured coating has good mechanical property and wave-absorbing property. The invention also provides a preparation method of the environment-friendly wave-absorbing coating.
Owner:AEROSPACE SCI & IND WUHAN MAGNETISM ELECTRON
Preparation method of Nalpha-fluorenylmethoxycarbonyl-glutamine tert-butyl ester
InactiveCN105254537AHigh yieldQuality improvementCarbamic acid derivatives preparationOrganic compound preparationHigh volume manufacturingOrganic solvent
The invention discloses a preparation method of Nalpha-fluorenylmethoxycarbonyl-glutamine tert-butyl ester and mainly solves the technical problems of complexity, long period, low yield, high cost and the like of an original technology. The preparation method comprises steps as follows: step one, gln and tert-butyl acetate are mixed, and h-gln-otbu is prepared under the action of perchloric acid; or, gln and z-cl are mixed, z-gln-oh is prepared and mixed with tert-butyl acetate, z-gln-otbu is prepared and subjected to catalytic hydrogenation in methyl alcohol, and h-gln-otbu is prepared; step two, h-gln-otbu and a fmoc-group protective agent are mixed, pH value is regulated to 8-9 by an alkali compound sodium carbonate aqueous solution in the presence of an organic solvent, fmoc-gln-otbu is prepared through reaction, and a pure product of fmoc-gln-otbu is prepared through processing. With the adoption of setting of a reasonable process route, Nalpha-fluorenylmethoxycarbonyl-glutamine tert-butyl ester and an intermediate thereof are prepared, and Nalpha-fluorenylmethoxycarbonyl-glutamine tert-butyl ester is applicable to mass production.
Owner:上海吉尔多肽有限公司 +1
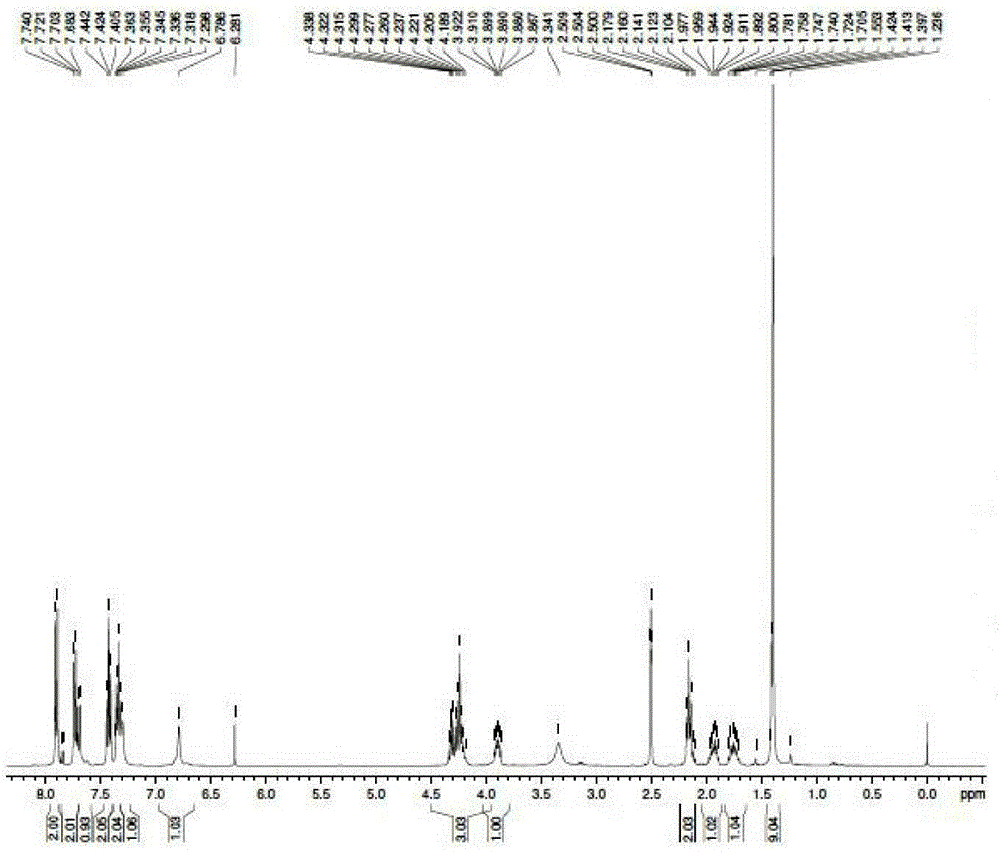
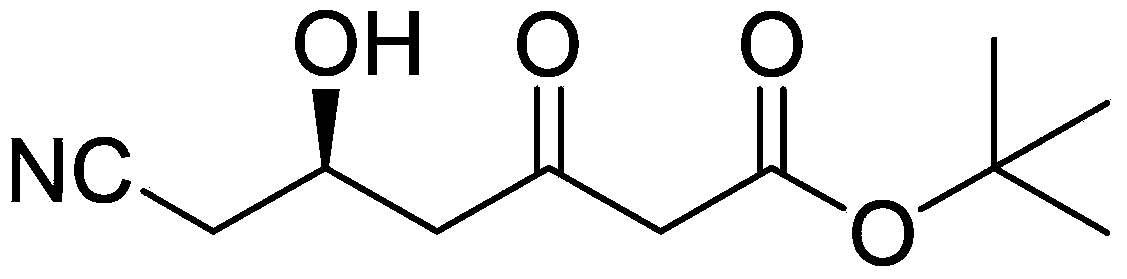
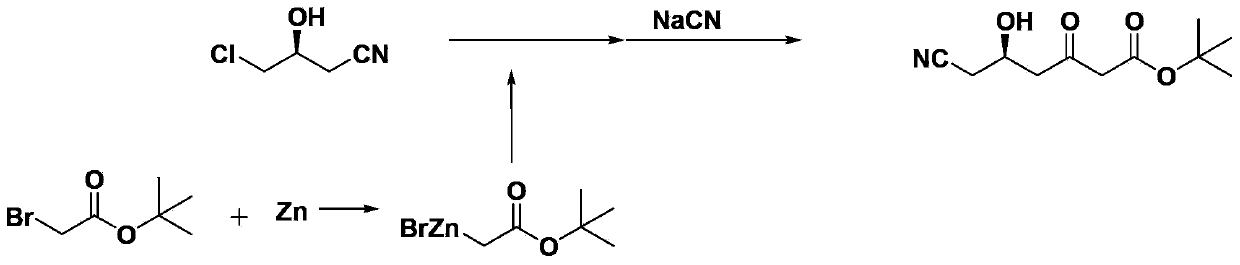
Method for preparing (5R)-6-cyanogroup-5-hydroxy-3-oxocaproic acid tert-butyl ester
ActiveCN103420871AReduce manufacturing costReduce usageCarboxylic acid nitrile preparationOrganic compound preparationBlaise reactionOrganic acid
The invention discloses a method for preparing (5R)-6-cyanogroup-5-hydroxy-3-oxocaproic acid tert-butyl ester and belongs to the technical field of medical drug chemical engineering. The method includes the following steps: adding organic acid, zinc powder and tert-butyl acetate in an organic solvent, and reacting at the temperature of 45-55 DEG C to obtain a tert-butyl acetate zinc reagent; adding (S)-4-chlorine-3-hydroxybutyronitrile in the tert-butyl acetate zinc reagent to perform Blaisereaction at the temperature of 65-70 DEG C, adding inorganic acid to adjust the pH value to be 5-7 after the reaction is completely performed, then adding a cyaniding reagent at the temperature of 45-55 DEG C to perform the cyanogroup replacement reaction, and after the reaction is completely performed, separating and purifying to obtain the (5R)-6-cyanogroup-5-hydroxy-3-oxocaproic acid tert-butyl ester, wherein the molar ratio of the (S)-4-chlorine-3-hydroxybutyronitrile, the tert-butyl acetate, the zinc power, the organic acid to the cyaniding reagent is 1:(1-2):(1.5-3): (0.05-0.2): (1.5-3).
Owner:湖北楚维药业有限公司
A kind of preparation technology of tert-butyl hydroquinone
ActiveCN105294403BReduce usageLow DTBHQ contentOrganic chemistryOrganic compound preparationWarm waterSolvent
The invention relates to the technical field of antioxidant preparation methods, and particularly relates to a preparation process of tert-butylhydroquinone (TBHQ). The preparation process comprises the following steps of (1) simultaneously adding excessive dilute sulphuric acid, equimolar hydroquinone and tertiary butyl acetate to a reaction still, wherein the weight ratio of the dilute sulphuric acid to the tertiary butyl acetate is 1:(3 to 6); (2) stirring and heating to 85 to 95 DEG C, and reacting for 1 to 8 hours; (3) stopping stirring, cooling to 65 to 75 DEG C, and then performing centrifugal filtration, wherein filtrate of sulphuric acid is recycled, and obtained filter residues are washed with 1 to 3 times warm water, so as to obtain a crude product of the TBHQ. Compared with the prior art, the preparation process has the advantages that the hydroquinone and the tertiary butyl acetate are used as raw materials, and the dilute sulphuric acid is used as a catalyst and a reaction solvent, so as to promote a reaction; tertiary butyl is supplied by the tertiary butyl acetate, so that side reactions are reduced; the crude product contains 70% to 75% of TBHQ, the content of DTBHQ (Di-Tert-Butylhydroquinone) as a by-product is low, the yield can reach 65 to 70% after purification, the yield is greatly increased, and great economic benefits are obtained.
Owner:东莞市感恩食品科技有限公司
Biological synthesis method of atorvastatin intermediate
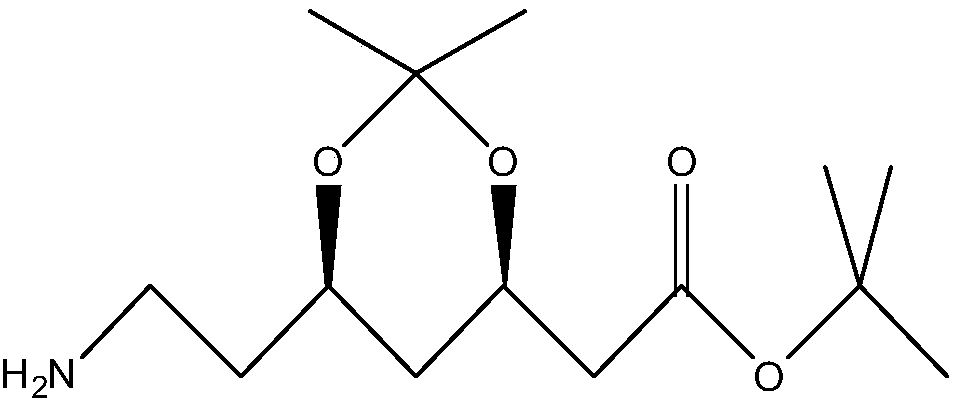
ActiveCN108315365AMild reaction conditionsNo pollution in the processFermentationChemical synthesisSynthesis methods
The invention discloses a biological synthesis method of an atorvastatin intermediate. The biological synthesis method comprises the following step: carrying out enzyme catalysis reaction on a compound (4R,6R)-6-(1-amino-1-carboxylethyl)-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate under the action of amino acid decarboxylase to generate a compound (4R,6R)-6-(aminoethyl)-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate, i.e., the atorvastatin intermediate. The biological synthesis method disclosed by the invention has moderate reaction conditions and no special requirements on equipment; a chemical synthesis method is combined with an enzyme method and pollution to the environment is not caused; reaction conditions are easy to control, the operation is simple and convenient and a technological flow is simple.
Owner:ZHEJIANG HONGYUAN PHARMA
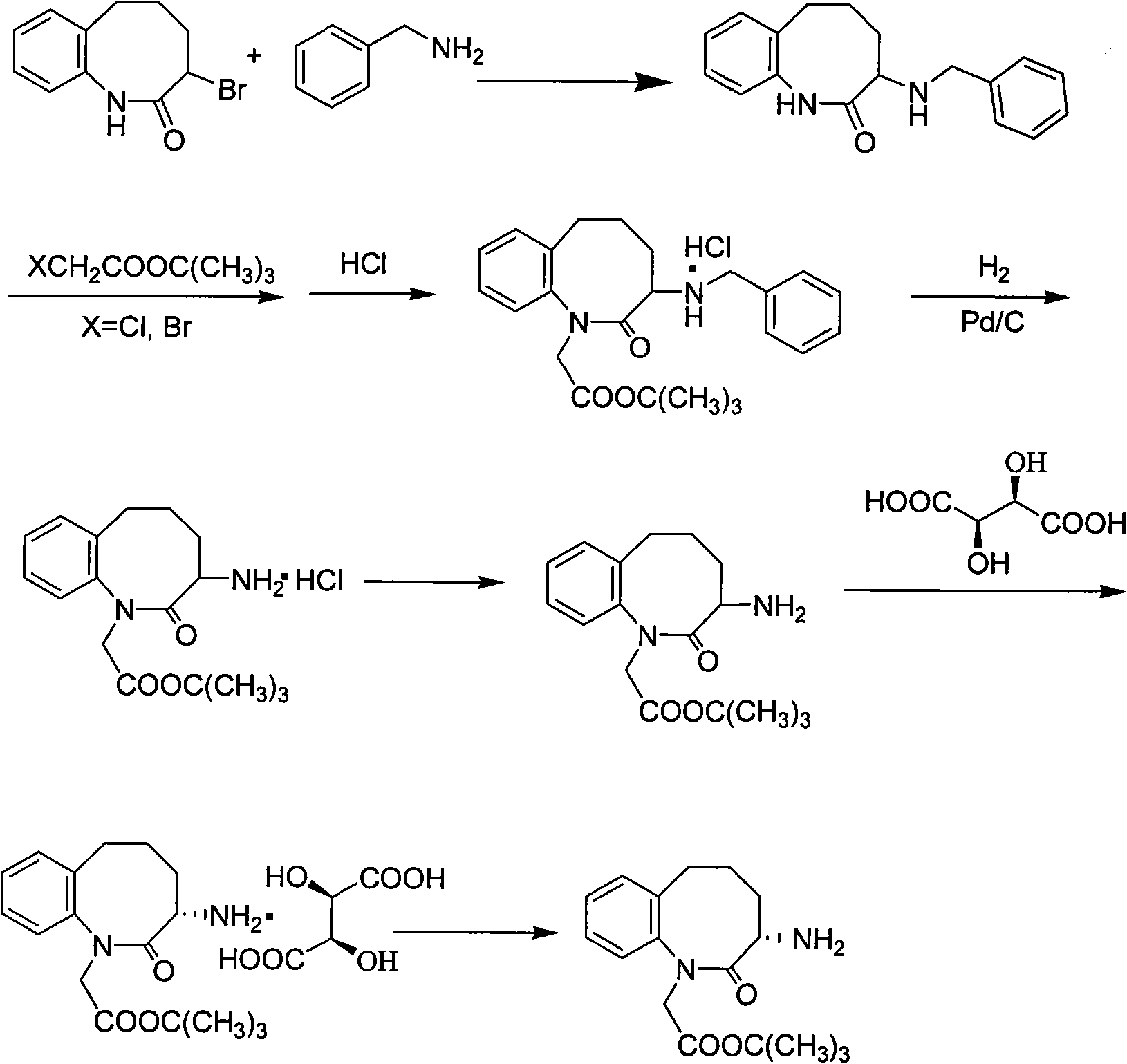
Method for preparing 3-(S)- amino-2,3,4,5-tetrahydro-2-oxy-1H-1-benzazepin-1-tert-butyl acetate
ActiveCN101628894AEasy to operateMild process conditionsOrganic chemistryHydrogenation reactionTertiary butyl acetate
The invention discloses a method for preparing 3-(S)- amino-2,3,4,5-tetrahydro-2-oxy-1H-1-benzazepin-1-tert-butyl acetate, which comprises the following steps: using 3-brom-1,3,4,5- tetrahydro-2H-1- benzazepin-2-ketone as raw material; carrying out condensation reaction with benzylamine and chloroacetic acid tert-butyl ester, HCl gas salt-forming reaction and hydrogenation reaction under the existence of Pd / C to obtain racemic 3-(S)-amino-2,3,4,5-tetrahydro-2-oxy-1H-1-benzazepin-1-tert-butyl acetate; separating the racemic body by L-(+)tartaric acid, and neutralizing to obtain the target products. The invention has the advantages of simple operation, mild and safe process condition, few three wastes and high yield. The products have high purity and bright color.
Owner:ZHEJIANG SANHE PHARMACHEM +1
Lacquer thinner
A lacquer or other coating thinner having a low volatile organic compound (VOC) rating which permits its use for cleaning and thinning in government regulated areas. The thinner has an acetone, methyl acetate or tertiary butyl acetate or mixture as a base. It has various non-hazardous ingredients which include a soy oil material, a dibasic ester and a glycol and carbonate ingredient such as tetrahydrofurfuryl alcohol.
Owner:BORTZ DISTRIBUTING CO
Method for preparing rosuvastatin calcium intermediate
ActiveCN104016961AReduce generationSimple processing methodOrganic chemistryTertiary butyl acetateRosuvastatin Calcium
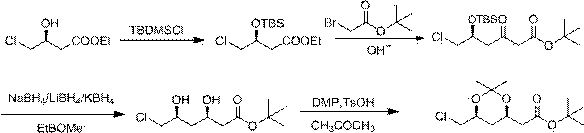
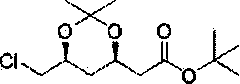
The invention discloses a method for preparing a rosuvastatin calcium intermediate. The name of the intermediate is 2-[(4R, 6S)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-yl]-tert-butyl acetate. The preparation method comprises the following steps: (1) preparing an intermediate I; (2) preparing an intermediate II; (3) preparing an intermediate III; and (4) preparing the 2-[(4R, 6S)-6-(chloromethyl)-2,2-dimethyl-1,3-dioxane-4-yl]-tert-butyl acetate. The method has the beneficial effects that active hydroxyl in an initial material is protected in the reaction process disclosed by the invention, side reaction is reduced, the technique is improved, the reaction efficiency is improved, and the method is applicable to large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Preparation method for varespladib
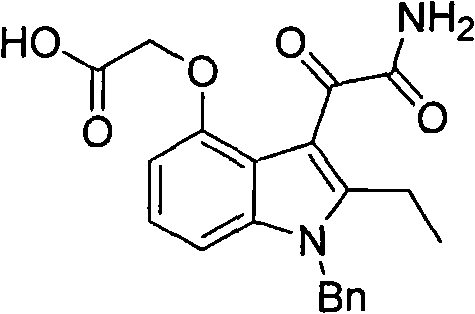
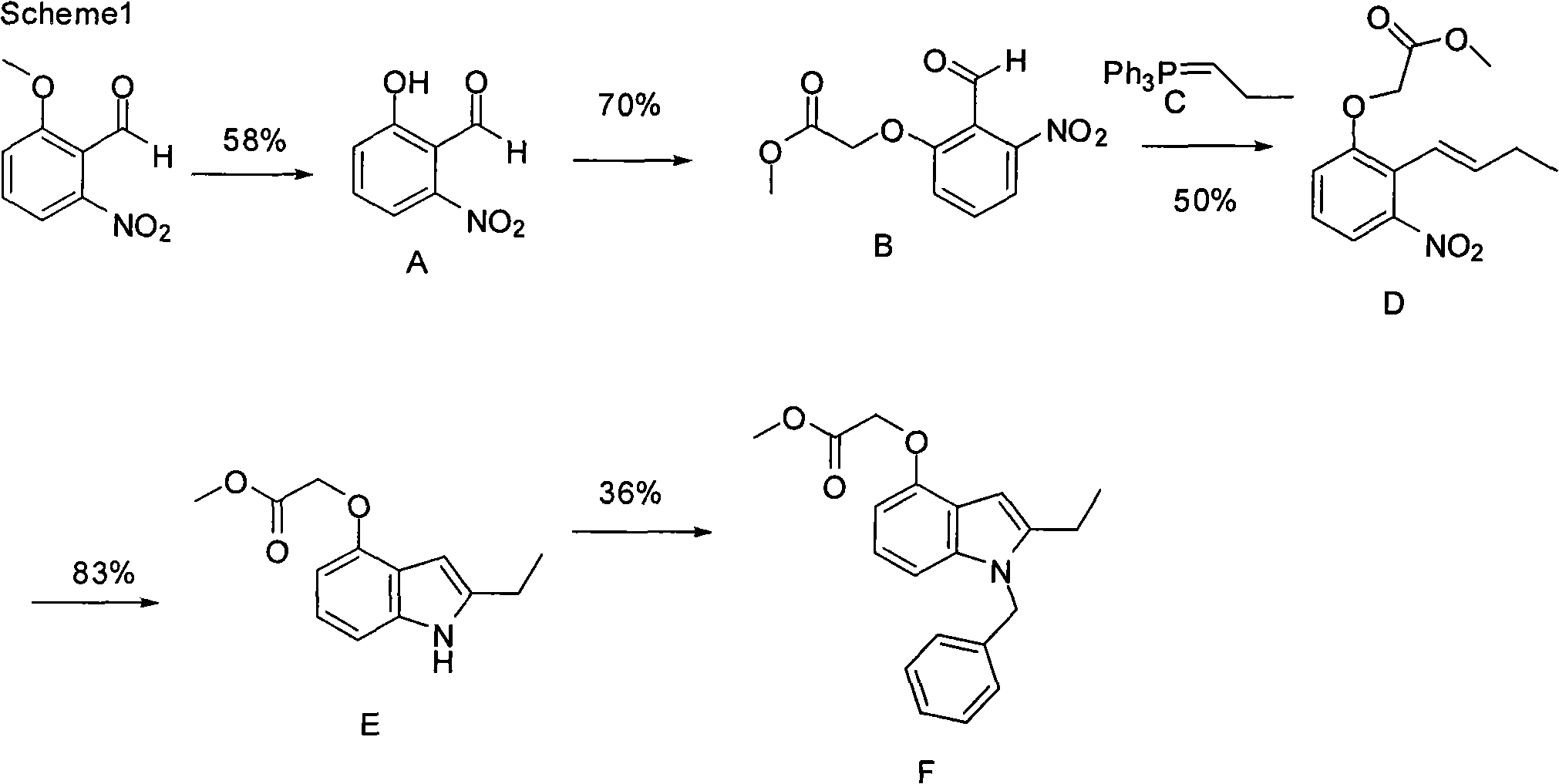
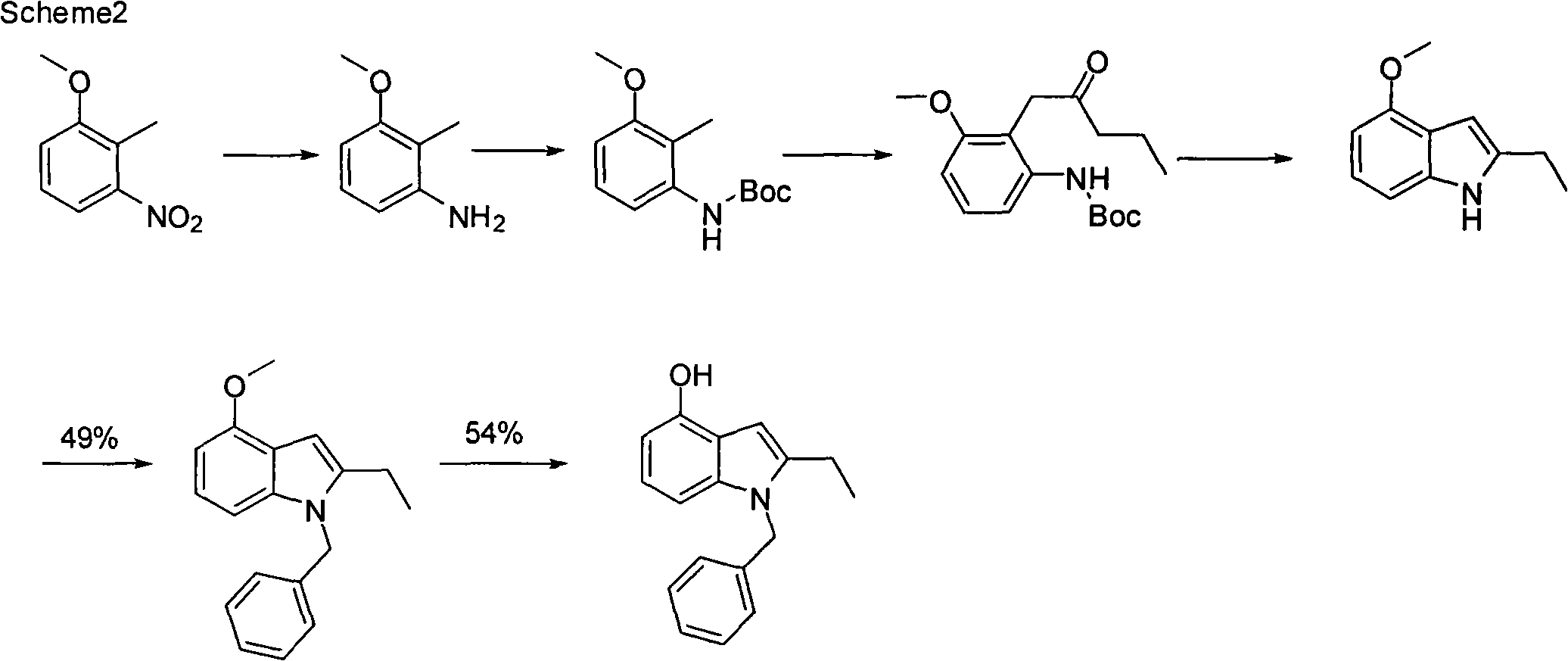
The invention discloses a preparation method for varespladib. 4-oxyindole is adopted as starting material, phenylsulfonyl is used for protecting the N atom after hydroxy group protection, so that an acetyl group is introduced to the site 2 of indole, 2-ethyl-4-oxyindole is obtained by reduction and deprotection, tert-butyl acetate is introduced to the hydroxyl group, the site 3 of the indole is acylated by oxalyl chloride and then ammonolyzed after N is protected by a benzyl group, and finally, tert-butyl ester is hydrolyzed, so that the varespladib is obtained. The invention has the advantages of cheap and easily-obtained starting material, mild reaction conditions, simple operation process, low cost and high yield.
Owner:ZENJI RES LAB
Pitavastatin calcium intermediate preparation method
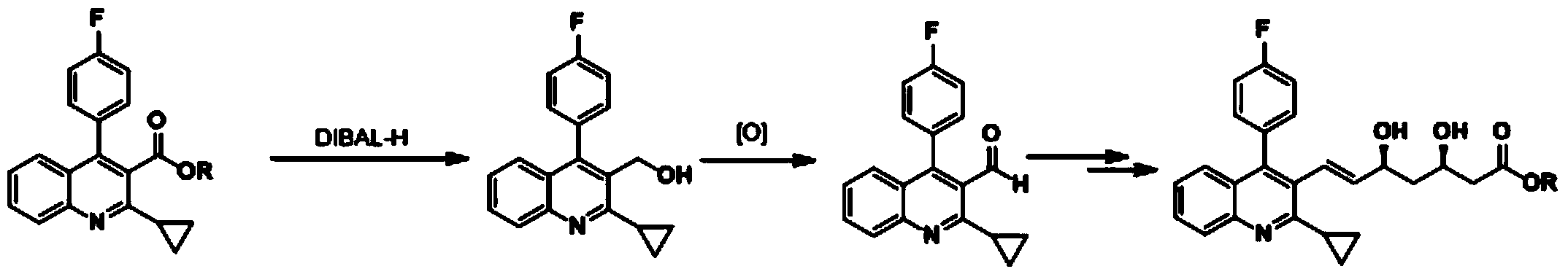
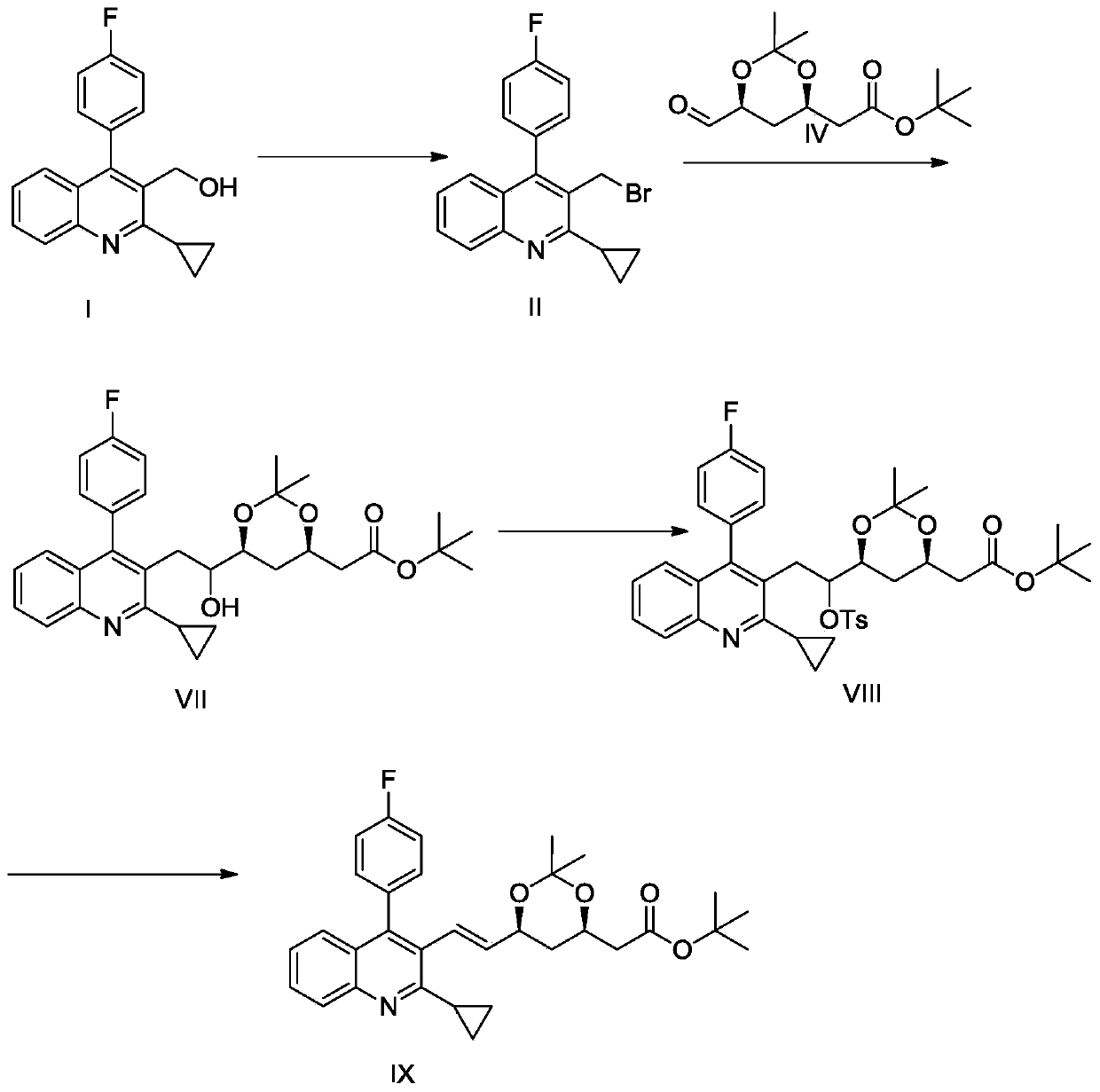
PendingCN110407818AAvoid it happening againImprove protectionOrganic chemistryPhosphorus tribromideQuinoline
The invention discloses a pitavastatin calcium intermediate preparation method, and relates to the technical field of preparation of pitavastatin calcium intermediates. In the prior art, the Wittig reaction can generate a large amount of solid waste triphenyl phosphorus oxychloride, and the solid waste is difficult to completely remove through post-treatment purification. A purpose of the presentinvention is to solve the problem in the prior art. The preparation method comprises: 1, carrying out a reaction on (2-cyclopropyl-4-(fluorophenyl)quinoline-3-yl)methanol I and phosphorus tribromide in dichloromethane to form 3-(bromomethyl)-2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline II, and extracting with dichloromethane; 2, carrying out a Reformatsky reaction on the 3-(bromomethyl)-2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline II and 2-((4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxane-4-yl)tert-butyl acetate to obtain an alcohol VII; and 3, adding p-toluenesulfonyl chloride in a dropwise manner to obtain p-toluenesulfonate VIII, treating the reaction liquid with potassium tert-butoxide, and carrying out an elimination reaction to obtain 2-((4R,6S)-6-((E)-2-(2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-yl)vinyl)-2,2-dimethyl-1,3-dioxane-4-yl) tert-butyl acetate V, or directly treating with a sodium carbonate aqueous solution to obtain the product V.
Owner:安庆恩聚生物医药科技有限公司
Far infrared anion nanometer solution
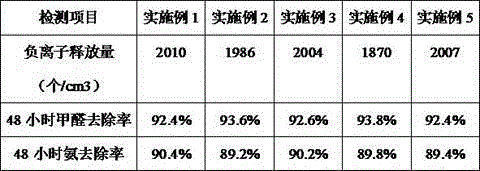
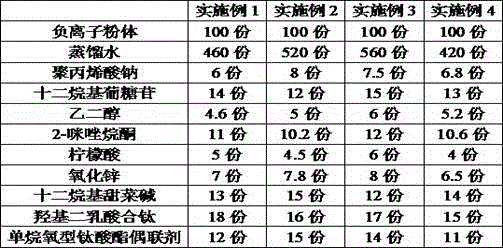
The invention discloses a far infrared anion nanometer solution. The far infrared anion nanometer solution is composed of the ingredients of, by weight, 100 parts of anion powder, 400 parts to 600 parts of distilled water, 6 parts to 8 parts of sodium polyacrylate, 12 parts to 15 parts of dodecyl polyglucoside, 4 parts to 6 parts of ethylene glycol, 10 parts to 12 parts of ethyleneurea, 4 parts to 6 parts of citric acid, 6 parts to 8 parts of zirconia, 12 parts to 15 parts of dodecyl dimethyl betaine, 15 parts to 18 parts of tert-butyl acetate, and 10 parts to 15 parts of alkoxy type titanate coupling agent. The ingredients are mixed and soaked for 2 days to 3 days, and then placed into a ball mill to be subjected to low-speed ball milling for 2 hours to 3 hours and high-speed ball milling for 40 minutes to 60 minutes, and the far infrared anion nanometer solution is obtained. According to the far infrared anion nanometer solution, a permanent nanoscale anion film is formed on the surface of a pollution source to decompose formaldehyde before formaldehyde escapes into the air, formaldehyde is prevented from causing harm to human bodies, anion release can be triggered only by slight changes of temperature and pressure without light irradiation under any room temperature states to decompose formaldehyde, and because the far infrared anion nanometer solution is colorless and odorless, the surfaces of furniture are not damaged.
Owner:昆山倍善环保实业有限公司
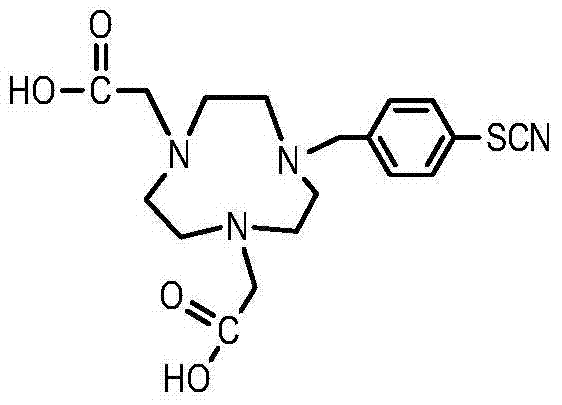
Method for synthesizing bifunctional chelating agent p-SCN-NODA (1,4,7-triazacyclooctane-1,4-diacetic acid-7-p-isothiocyanobenzyl)
InactiveCN103787998ASynthetic Method AdvantagesLow costOrganic chemistryBulk chemical productionAcetic acidBenzoyl bromide
The invention discloses a method for synthesizing a bifunctional chelating agent p-SCN-NODA ((1,4,7-triazacyclooctane-1,4-diacetic acid-7-p-isothiocyanobenzyl)). The method comprises the following steps: reacting 1,4,7-triazacyclooctane I (serving as a raw material) with tert-butyl bromoacetate in an organic solution, reacting an obtained product 1,4,7-triazacyclooctane-1,4-di-tert-butyl acetate II with 4-nitro-benzyl bromide to obtain a product 1,4,7-triazacyclooctane-1,4-di-tert-butyl acetate-7-p-nitrobenzyl III, performing decarboxylation protection on the product III in trifluoroacetic acid to obtain a product 1,4,7-triazacyclooctane-1,4-diacetic acid-7-p-nitrobenzyl IV, reducing in the presence of H2 to obtain an amino product 1,4,7-triazacyclooctane-1,4-diacetic acid-7-p-aminobenzyl V, and finally reacting the product V in the presence of SCCl2 to prepare the bifunctional chelating agent p-SCN-NODA. According to the method, the operation is easy, the cost is low, and the quality is stable and controllable.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com