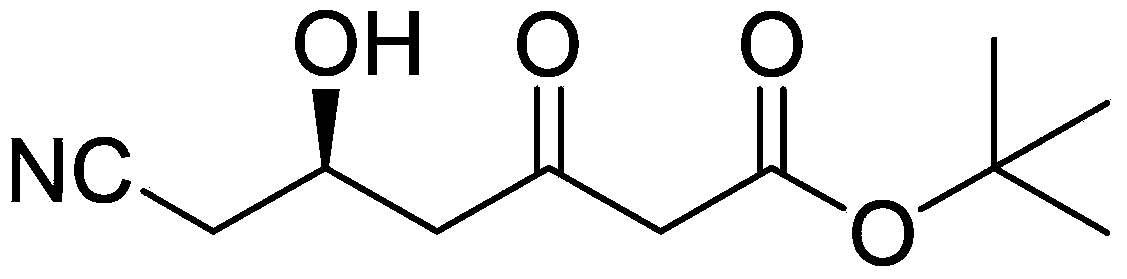
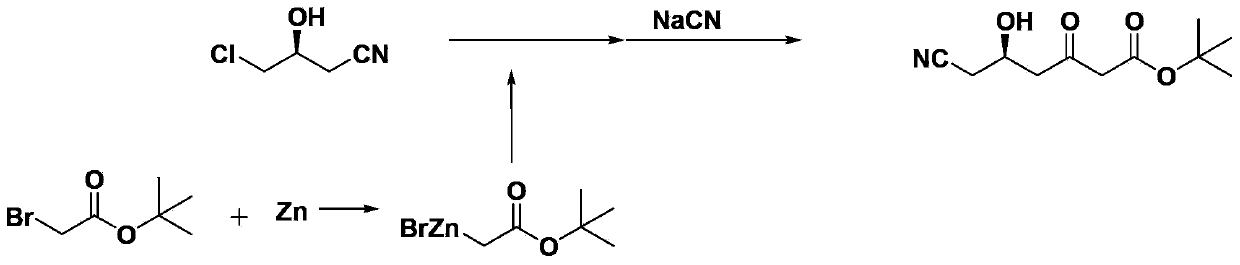
Method for preparing (5R)-6-cyanogroup-5-hydroxy-3-oxocaproic acid tert-butyl ester
A technology of tert-butyl hexanoate and tert-butyl bromoacetate, which is applied in the preparation of atorvastatin intermediates and the field of preparation of tert-butyl-6-cyano-5-hydroxy-3-oxohexanoate, can Solve the problems that are not suitable for industrial production, difficult to control in terms of safety, and high cost of industrialization, and achieve the effects of mild reaction conditions, reduced production costs, and reduced production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1. Add 300 mL of anhydrous tetrahydrofuran, 97.5 g of zinc powder and 7.2 g of methanesulfonic acid into the reaction flask, and heat and stir at 65° C. for 1 hour. At 45°C, 214.5 g of tert-butyl bromoacetate was slowly added dropwise, followed by mixing and stirring for 1 hour.
[0029] 2. 119.5 g of (S)-4-chloro-3-hydroxybutyronitrile was slowly added dropwise, and after the dropwise addition was completed, the temperature was raised to 65° C. and maintained for 3 hours. After cooling to 0°C, a hydrochloric acid solution with a mass concentration of 10% was added dropwise, the pH was adjusted to 6-7, the temperature was raised to 20°C, and the mixture was stirred for 1 hour.
[0030] 3. Slowly add 326.7 g of sodium cyanide solution with a mass concentration of 30% dropwise, and stir vigorously for 3 hours at 50°C. The tetrahydrofuran was distilled off under reduced pressure, 200 mL of ethyl acetate was added for extraction, and the layers were separated. The aqueous ...
Embodiment 2
[0032] 1. Add 250 mL of 1,2-dichloroethane, 130 g of zinc powder and 12.2 g of benzoic acid into the reaction flask, and heat and stir at 50° C. for 1 hour. At 55°C, 234 g of tert-butyl bromoacetate was slowly added dropwise, followed by mixing and stirring for 1 hour.
[0033] 2. 119.5 g of (S)-4-chloro-3-hydroxybutyronitrile was slowly added dropwise, and after the dropwise addition was completed, the temperature was raised to 65° C. and maintained for 4 hours. After cooling to 0°C, a nitric acid solution with a mass concentration of 20% was added dropwise, the pH was maintained at 5-6, the temperature was raised to 20°C, and stirred for 1 hour.
[0034] 3. Slowly add 245 g of sodium cyanide solution with a mass concentration of 30% dropwise, and stir vigorously for 4 hours at 55°C. The 1,2-dichloroethane was distilled off under reduced pressure, 150 mL of ethyl acetate was added for extraction, and the layers were separated. The aqueous layer was extracted again with 150 m...
Embodiment 3
[0036] 1. Add 300 mL of anhydrous methyl tert-butyl ether, 98 g of zinc powder, and 14.2 g of p-toluenesulfonic acid into the reaction flask, and heat and stir at 65° C. for 1 hour. At 45°C, 222 g of tert-butyl bromoacetate was slowly added dropwise, followed by mixing and stirring for 1 hour.
[0037] 2. 119.5 g of (S)-4-chloro-3-hydroxybutyronitrile was slowly added dropwise, and after the dropwise addition was completed, the temperature was raised to 65° C. and maintained for 3 hours. After cooling to 0°C, a phosphoric acid solution with a mass concentration of 15% was added dropwise, the pH was maintained at 6-7, the temperature was raised to 20°C, and the mixture was stirred for 1 hour.
[0038] 3. Slowly add 1299.9 g of potassium cyanide solution with a mass concentration of 10% dropwise, and stir vigorously for 5 hours at 50°C. The methyl tert-butyl ether was distilled off under reduced pressure, 200 mL of ethyl acetate was added for extraction, and the layers were sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com