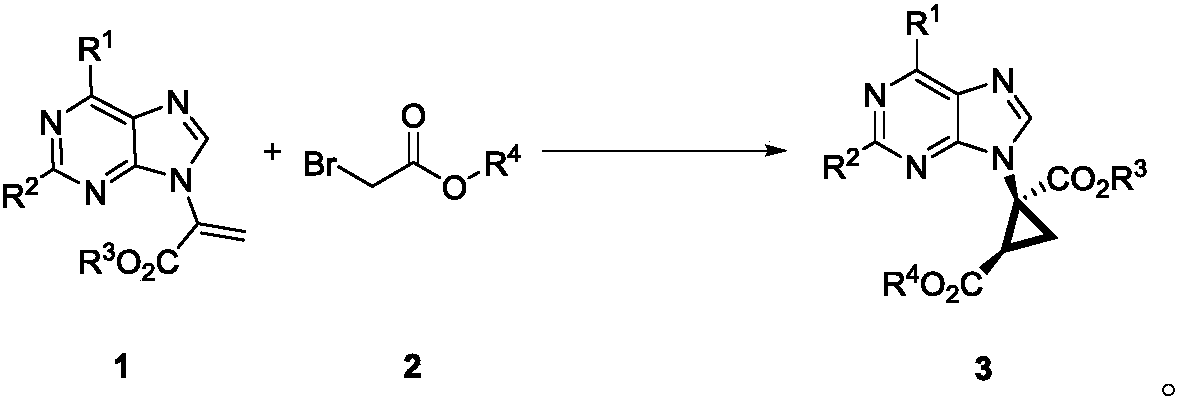
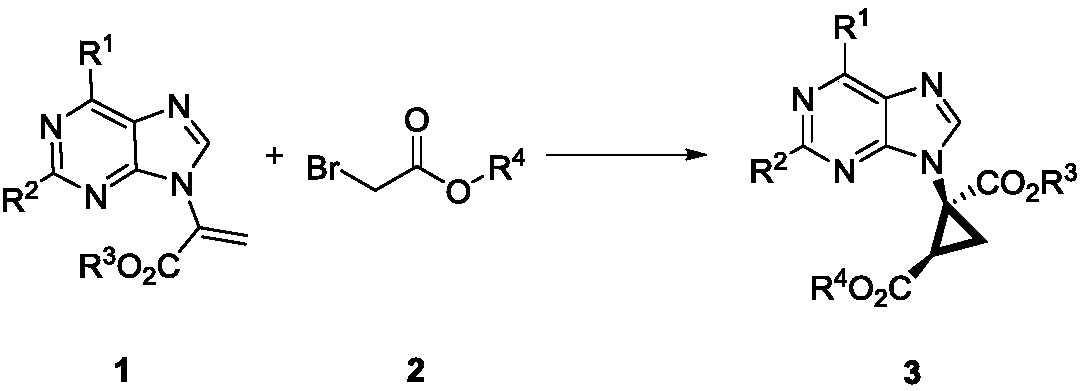
Method for synthesizing chiral ternary carbocyclic nucleoside through asymmetric cyclopropanation triggered by Michael addition
A technology for the synthesis of three-membered carbocycles, which is applied in organic chemistry and other fields, can solve the problems of high synthesis cost and cumbersome methods of cyclopropane nucleoside derivatives, and achieves efficient synthesis methods, rich product structures, and high stereoselectivity of products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021]
[0022]
[0023] a Unless otherwise stated, the reaction conditions were as follows: α-purine-substituted acrylate 1a (0.1 mmol), tert-butyl bromoacetate 2c (0.11 mmol), quinine-based catalyst (10 mol%) and base (1.1 equivalents) in 1 mL of solvent in 0°C reaction. b Separation yield. c Determined by chiral HPLC analysis. d React at room temperature. e Increasing the amount of base to 2 equivalents increased the yield to 52%, ee=92%. f The amount of catalyst used is 5 mol%. g at -10°C. h at -20°C.
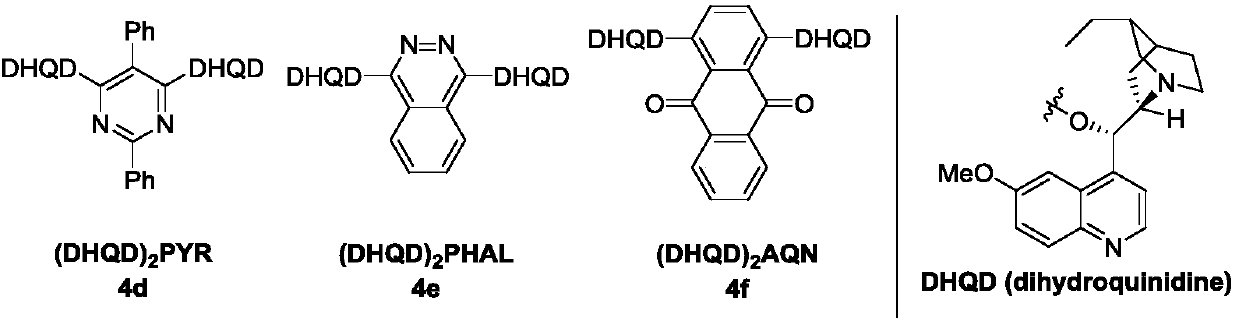
[0024] In the process of screening the reaction conditions, the influence of amine catalysts on the reaction was firstly investigated (markers 1-6). At the same time, by comparing the effects of different catalysts on the reaction, the best catalyst for catalyst 4f was determined.
[0025] Investigation of reaction conditions: In a 10mL vacuum tube, add α-purine substituted 6-Cl ethyl acrylate 1a (25.2mg, 0.1mmol), (DHQD) 2 AQN (8.6 mg, 10 mol%), ...
Embodiment 2
[0038] In a 10 mL vacuum tube, α-purine substituted 6-dimethylaminoacrylate (26.1 mg, 0.1 mmol), (DHQD) 2 AQN (8.6 mg, 10 mol%) and tert-butyl bromoacetate (17 μL, 0.11 mmol). Then 0.66 mL of dichloromethane and 0.34 mL of acetonitrile were added. Seal the reaction tube, and place the reaction tube in a cryopump at 0°C for 2 days. The reaction was tracked by TLC. After the reaction was terminated, the reaction solution was concentrated in vacuo, and then the target compound 3fc was obtained by column chromatography with a yield of 84%, 97% ee.
Embodiment 3
[0040] In a 10 mL vacuum tube, α-purine substituted 6-diethylaminoacrylate (28.9 mg, 0.1 mmol), (DHQD) 2 AQN (8.6 mg, 10 mol%) and tert-butyl bromoacetate (17 μL, 0.11 mmol). Then 0.66 mL of dichloromethane and 0.34 mL of acetonitrile were added. Seal the reaction tube, and place the reaction tube in a cryopump at 0°C for 2 days. The reaction was followed by TLC. After the reaction was terminated, the reaction solution was concentrated in vacuo, and then the target compound 3gc was obtained by column chromatography with a yield of 87%, 94% ee.
[0041] Representative compound characterization data are as follows:
[0042]3gc colorless oily liquid, 87% yield, 94% ee.[α] 25 D =-80.1 (c=1.2, CH 2 Cl 2 ); Ee value is detected by chiral HPLC (mobile phase, n-hexane / 2-propanol=80 / 20, flow rate: 0.6mL / min, detection wavelength: 250nm, retention time: 13.924min, 18.512min.); 1 H NMR (600MHz, CDCl 3 ):8.30(s,1H),7.66(s,1H),4.13-4.16(m,2H),3.96(br,4H),2.98(br,1H),2.05-2.45(m,2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com