Process for preparing and extracting tert-butyl acetate
A technology of tert-butyl acetate and acetic acid, applied in the field of preparation and purification of tert-butyl acetate, can solve problems such as difficulty in separation and purification of tert-butyl acetate, ineffectiveness, etc., achieve continuous esterification reaction and separation and purification, reduce production costs, overcome The effect of difficult water separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
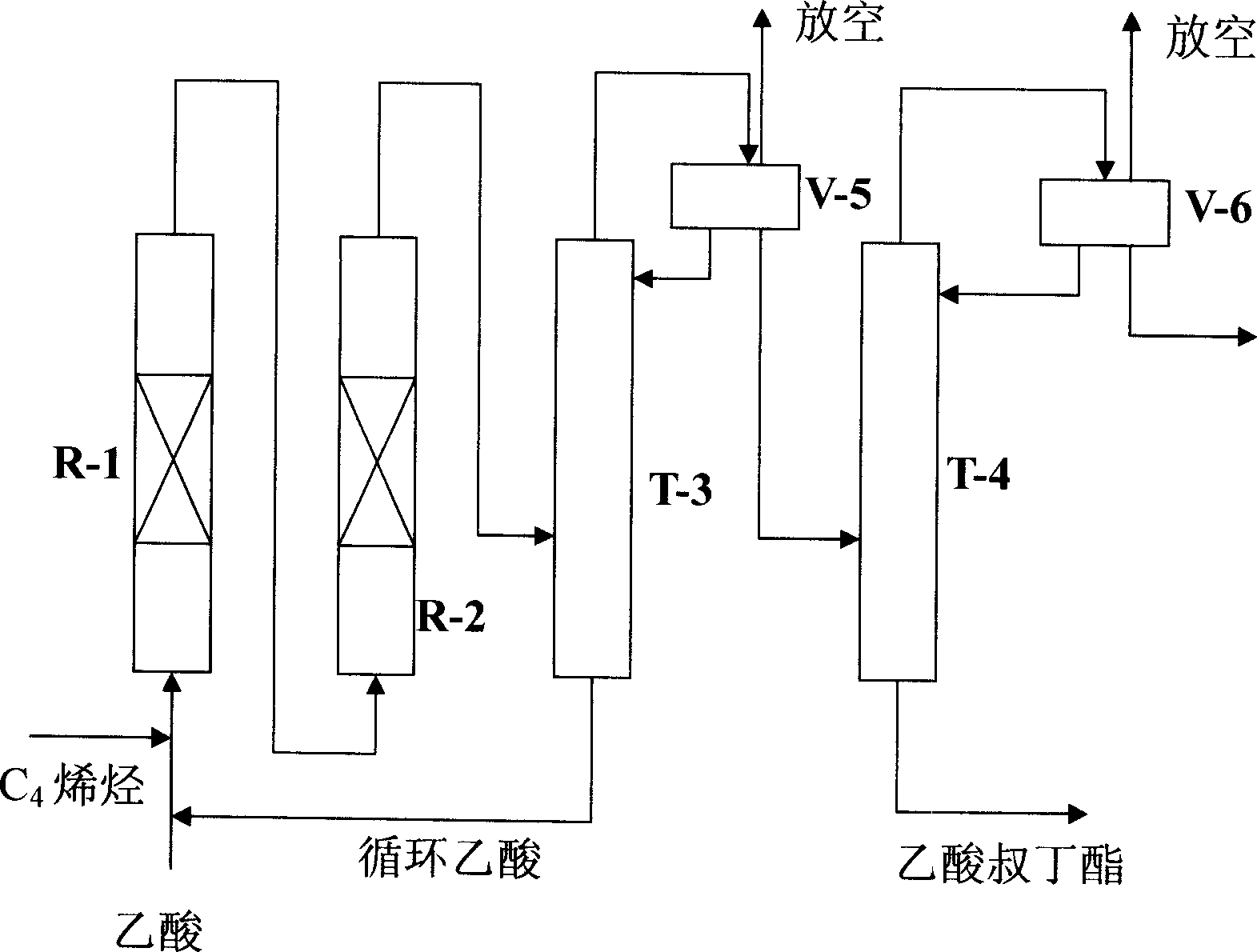
[0017] Acetic acid and C 4 The reaction is an acid-catalyzed reaction, which can be a batch or continuous operation mode. The inner diameter of the reaction tube is 16mm, the catalyst adopts a strong acid type ion exchange resin, the catalyst filling amount is 30ml~90ml, the acetic acid is metered in with a flat flow pump, and temperature control equipment is used for temperature control. The raw materials used in experiments T-1, T-2, T-3, and T-4 are acetic acid and C 4 , Acetic acid is fed into the esterification tower equipped with ion exchange resin catalyst at a certain rate to maintain a stable C 4 The exhaust gas flow rate (60ml / min), the pressure is 0.8MPa, the reaction temperature is changed, and the reaction takes 6-7 hours to sample for analysis. The crude ester is deacidified at 85-100°C, dehydrocarbonized at 70-85°C, dealcoholized at 78-90°C, and finally rectified at 90-100°C to obtain tert-butyl acetate product. The results are shown in Table 1.
[0018] ...
Embodiment 2
[0020] The raw materials used in experiments T-5, T-6, T-8, T-10, T-11 are acetic acid, water and C 4 , The raw materials used in experiment T-7 are acetic acid, alcohol and C 4 , Experiment T-9 uses acetic acid and C 4 As the raw material, keep the reaction temperature at 65°C, change the acetic acid space velocity and the reaction pressure, and other conditions are the same as in Example 1. The crude ester is deacidified at 85-100°C, dehydrocarbonized at 70-85°C, dealcoholized at 78-90°C, and finally rectified at 90-100°C to obtain tert-butyl acetate product. The results are shown in Table 2.
[0021] Test number
Embodiment 3
[0023] The crude tert-butyl acetate product (containing 18.97% tert-butyl acetate) obtained from the first esterification tower is recycled into the second esterification tower, and supplemented C 4 Esterification reaction occurs, control C 4 The exhaust gas flow rate is 60ml / min. Keep the reaction temperature at 65°C, change the reaction pressure and the injection space velocity of the crude product of tert-butyl acetate to perform experiments T-12, 13, 14, 15, 16, and other conditions are the same as in Example 1, and take samples for analysis after 3 hours of reaction. The crude ester is deacidified at 85-100°C, dehydrocarbonized at 70-85°C, dealcoholized at 78-90°C, and finally rectified at 90-100°C to obtain tert-butyl acetate product. The results are shown in Table 3.
[0024] Test number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com