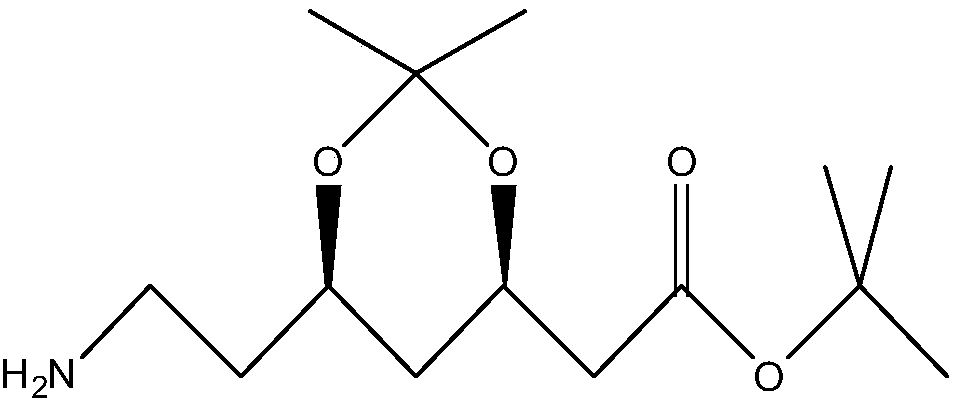
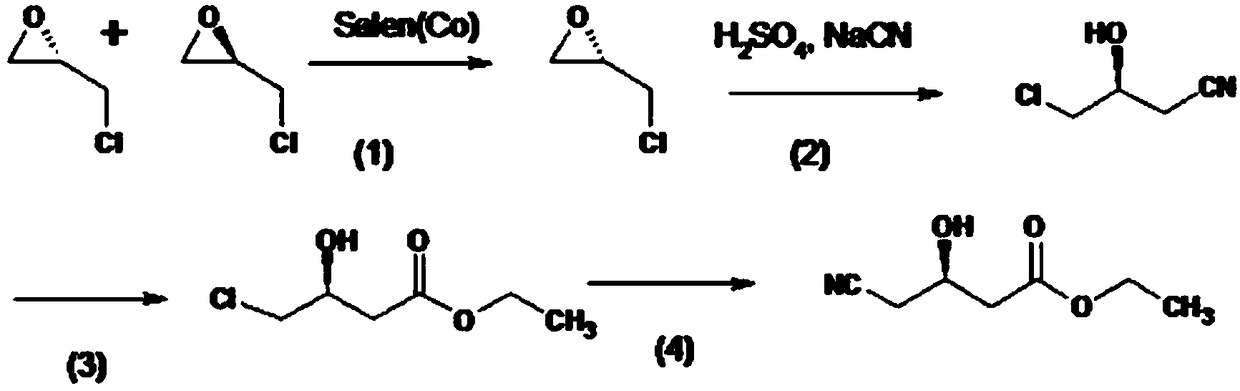
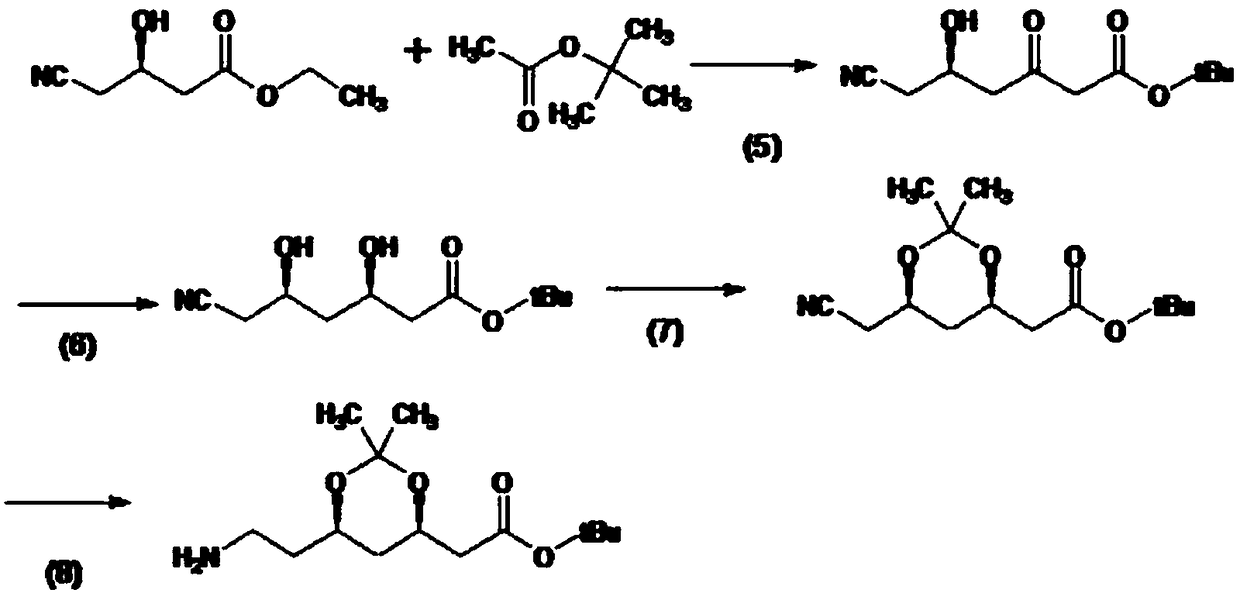
Biological synthesis method of atorvastatin intermediate
A biosynthetic technology of atorvastatin, which is applied in the field of preparation of raw materials and pharmaceutical intermediates, can solve the problems of unsuitability for industrialization, many by-products, long process flow, etc., and achieve easy control of reaction conditions, mild reaction conditions, The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Preparation of aldolase
[0042] Recombinant aldolase genetically engineered bacteria, the specific preparation method is: select the amino acid sequence of the aldolase derived from Escherichia coli, carry out artificial design, and synthesize the artificially designed sequence through the whole gene (commissioned by GenScript Biotechnology Co., Ltd. ), cloned into the Nde I and Xho I restriction sites of the expression vector pET28a, and transformed the host bacteria E.coliBL21 (DE3) competent cells; after picking the positive transformant and identifying it by sequencing, the recombinant expression vector was obtained; the recombinant expression vector Transform into E. coli BL21 (DE3) strain, obtain the recombinant aldolase gene engineering bacteria that can induce the expression of recombinant aldolase.
[0043] Inoculate the recombinant aldolase genetically engineered bacteria into LB medium containing kanamycin, and culture overnight at 37°C to obtain seed cul...
Embodiment 2
[0054] Formula VI compound (S)-6-tert-butoxy-4-hydroxyl-2,6-dicarbonylhexanoic acid is carried out by formula VII compound 3-carbonyl propionate tert-butyl ester and pyruvate under the effect of aldolase Catalyzed reaction generates, and the reaction formula is as follows:
[0055]
[0056] The specific reaction process is as follows: in a 500mL shake flask, the formula VII compound 3-oxopropionate tert-butyl ester (10g, 69.44mmol) was dissolved in 40mL of methanol, then pyruvic acid (17.6g, 0.200mol) was added in the shake flask, Add the aldolase of 160mL phosphate buffered saline solution, 40g again, the preparation of aldolase is as embodiment 1, add the PLP of 1mM again, the MgCl of 20mM 2 , control the pH value of the reaction system to be 7.5, react in a shaker for 14h, then extract the organic phase with ethyl acetate, and rotate to evaporate to obtain an oily liquid. The product is detected by gas chromatography, and the product formula VI compound (S)-6- The conce...
Embodiment 3
[0058] Formula V compound (S)-3-hydroxyl-1-carbonyl valeric acid tert-butyl ester is decarboxylated by formula VI compound (S)-6-tert-butoxy-4-hydroxyl-2,6-dicarbonylhexanoic acid Under the action of enzymes, the enzyme-catalyzed reaction is generated, and the reaction formula is as follows:
[0059]
[0060] The specific reaction process is as follows: in a 500mL shake flask, the formula VI compound (S)-6-tert-butoxy-4-hydroxyl-2,6-dicarbonylhexanoic acid (20g, 86.21mmol) was dissolved in 20mL of DMSO , then add 180mL phosphate buffer solution in the shake flask, then add 30g of ketoacid decarboxylase in the shake flask, the preparation of ketoacid decarboxylase is as in Example 1, then add 1mM TPP, 20mM MgCl 2 , control the pH value in the reaction system to be 8, control the temperature in the reaction system to be 6°C, react in a shaker for 22h, purify, and pass 1 H-NMR and 13 C-NMR and MS confirmed that the product V compound (S)-tert-butyl 3-hydroxy-1-oxopentanoate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com