Preparation method of Nalpha-fluorenylmethoxycarbonyl-glutamine tert-butyl ester
A fluorene methoxycarbonyl and glutamine technology, applied in the field of peptide synthesis, can solve the problems of long cycle, low cost and high cost, and achieve the effects of improving quality, shortening cycle and simplifying process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
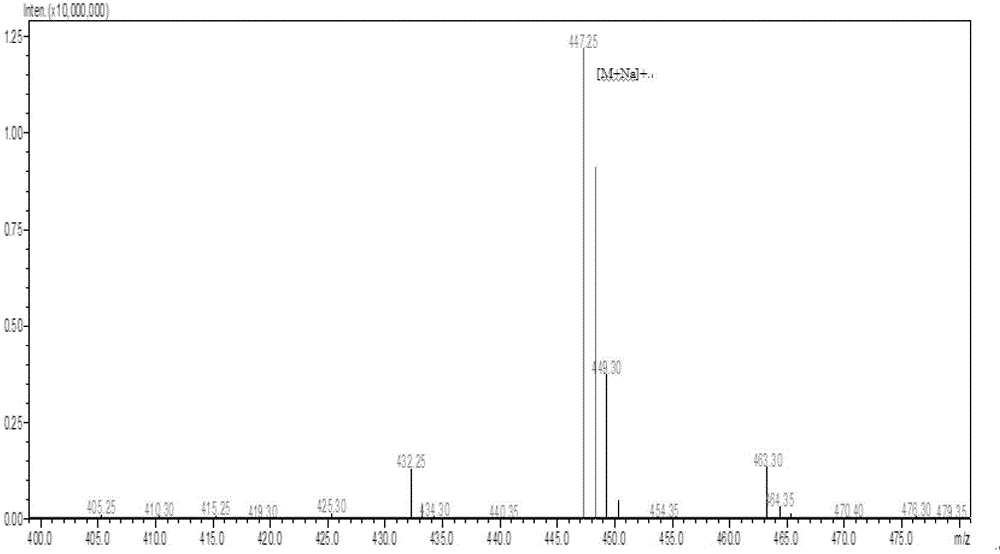
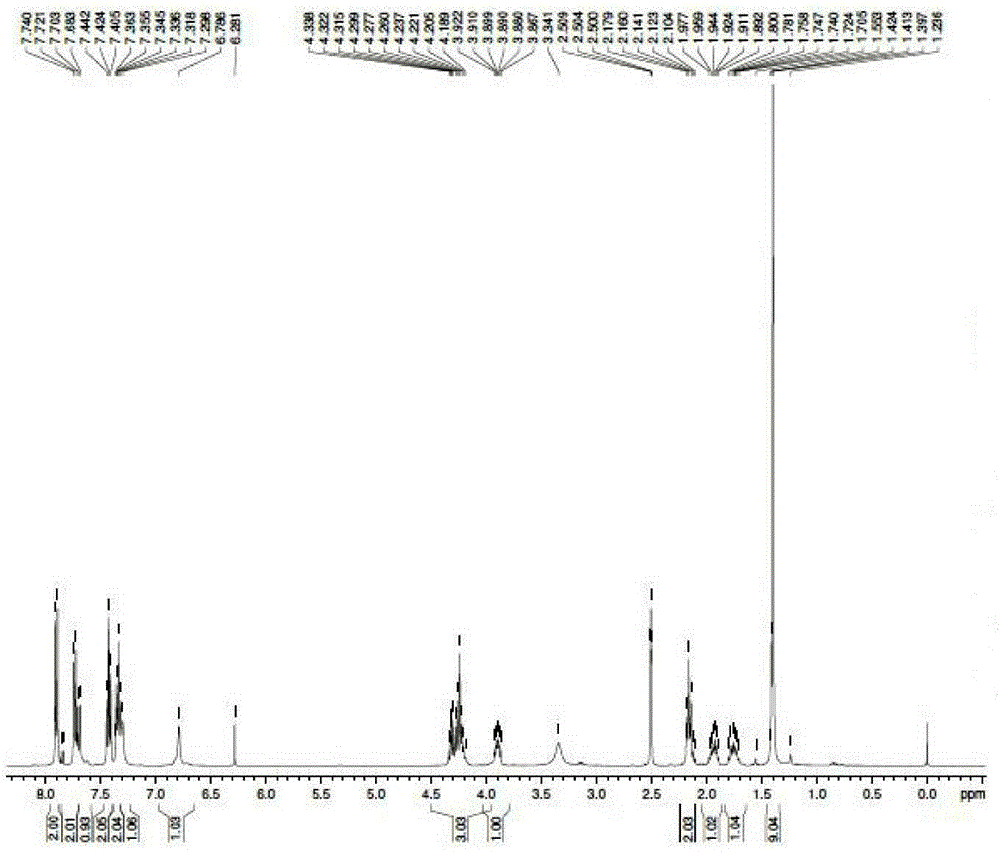
[0038] Add 795g of tert-butyl acetate to a 2000ml three-necked flask, then add 100ggln, stir, cool down to below 5°C, add 103.1g of perchloric acid dropwise, react at 20°C for 36 hours, then add 400ml of water, use sodium hydroxide Adjust the pH value of the system to about 8, and extract the water phase product once with 500ml tert-butyl acetate, combine the product phases and wash twice with saturated brine, and then concentrate to dryness to obtain 35g of oil, which is h-gln-otbu, the yield : 22.8%.
[0039] Add 35gh-gln-otbu to a 2000ml three-necked flask, then add 58.3g of fmoc-osu and 250ml of acetone, adjust the pH of the system to 8-9 with aqueous sodium carbonate, react at 15°C for 6 hours, and monitor the reaction by TLC process, after the reaction is completed, wash the system 4 times with a mixed solvent of 500ml of ethyl acetate and petroleum ether, then extract with 1L of ethyl acetate, acidify with 100g of citric acid and wash with acid water 3 times, and then w...
Embodiment 2
[0042] Add 298.2g tert-butyl acetate into a 2000ml three-necked flask, then add 75ggln, stir, cool down to below 5°C, add 67.04g perchloric acid dropwise, react at 15°C for 24 hours, then add 400ml of water, and oxidize with hydrogen The pH value of the sodium-adjusted system is about 8, and the aqueous phase product is extracted once again with 500ml tert-butyl acetate, and the combined product phase is washed twice with saturated brine, and then concentrated to dryness to obtain 30g of oil, which is h-gln-otbu, which is collected Rate: 26.2%.
[0043] Add 30gh-gln-otbu into a 2000ml three-necked flask, then add 62.6g of fmoc-osu and 250ml of THF, adjust the pH value of the system to 8-9 with aqueous sodium carbonate solution, react for 8 hours, and track and monitor the reaction process by TLC. After completion, the system was washed 3 times with a mixed solvent of 500ml of ethyl acetate and petroleum ether, then extracted with 1L of ethyl acetate, acidified with 80g of citr...
Embodiment 3
[0046] Add 788.6g tert-butyl acetate into a 2000ml three-necked flask, then add 66.1ggln, stir, cool down to below 5°C, add 81.8g perchloric acid dropwise, react at 25°C for 36 hours, then add 400ml of water, use Sodium hydroxide adjusts the pH value of the system to about 8, and then extracts the water phase product once with 500ml tert-butyl acetate, combines the product phase and washes it twice with saturated brine, and then concentrates to dryness to obtain 31g of oil, which is h-gln-otbu , Yield: 30.6%.
[0047] Add 31gh-gln-otbu to a 2000ml three-necked flask, then add 77.56g of fmoc-osu and 290ml of dioxane, adjust the pH of the system to 8-9 with aqueous sodium carbonate solution, react for 10 hours, and track and monitor the reaction by TLC process, after the reaction is completed, wash the system 4 times with a mixed solvent of 500ml of ethyl acetate and petroleum ether, then extract with 1L of ethyl acetate, acidify with 90g of citric acid and wash with acid water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com