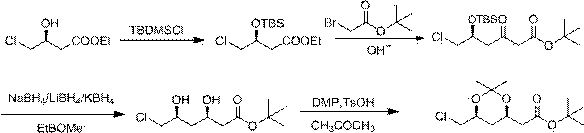
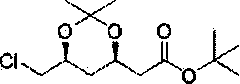
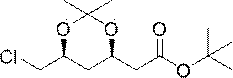Method for preparing rosuvastatin calcium intermediate
A technology for rosuvastatin calcium and its intermediates, which is applied in the field of drug synthesis, can solve problems that are not conducive to large-scale production, and achieve the effects of reducing side reactions, improving process methods, and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of intermediate Ⅰ: Dissolve 30g of ethyl S(-)-4-chloro-3-hydroxybutyrate in 300mL of tetrahydrofuran, cool down to 5°C, add 36.5g of triethylamine dropwise; after dropping, stir for 15 minutes ; Add 40.5g of tert-butyldimethylsilyl chloride dropwise at 5°C. After the drop is complete, heat up to 30°C for reaction, and monitor the reaction by TLC; The organic phase and the aqueous phase were extracted with toluene (500mL×3); the organic phases were combined, washed with saturated aqueous sodium chloride solution (500mL×2), dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain 49.1g of an oily product. Yield: 97%.
[0031] Preparation of intermediate II: under anhydrous and oxygen-free conditions, put 125mL of butyllithium (2M) solution in 250mL of tetrahydrofuran at -78°C, stir, and slowly add 29.1g of tert-butyl bromoacetate dropwise; Stir the reaction for 30 minutes; dissolve 35g of intermediate I in 100mL of tetrahydr...
Embodiment 2
[0035] Preparation of Intermediate I: Dissolve 20g of S(-)-4-chloro-3-hydroxybutyric acid ethyl ester in 300mL of toluene, cool down to 5°C, add 42.2g of HMDS dropwise; after dropping, stir the reaction for 15 minutes; Add 36g of tert-butyldimethylsilyl chloride dropwise at 5°C. After the drop is complete, heat up to 30°C for reaction, and monitor the reaction by TLC; The aqueous phase was extracted with toluene (300mL×3); the organic phases were combined, washed with saturated aqueous sodium chloride solution (300mL×2), dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain 33.3g of an oily product. Yield: 95%.
[0036] Preparation of Intermediate II: Under anhydrous and oxygen-free conditions, put 107mL of sodium hexamethyldisilazide (2M) solution in 200mL of toluene at -78°C, stir, and slowly add 27g of tert-butyl bromoacetate dropwise; After dropping, stir and react for 30 minutes; dissolve 30g of intermediate I in 90mL of toluene and add d...
Embodiment 3
[0040] Preparation of intermediate Ⅰ: Dissolve 35g of S(-)-4-chloro-3-hydroxybutyric acid ethyl ester in 500mL of dichloromethane, cool down to 5°C, add 54.4g of DIPEA dropwise; after dropping, stir for 15 minutes ; Add 79.1g of tert-butyldimethylsilyl chloride dropwise at 5°C, after dropping, heat up to 30°C for reaction, and monitor the reaction by TLC; after the reaction is completed, pour the reaction solution into 1200mL ice-water solution, stir and separate The organic phase and the aqueous phase were extracted with dichloromethane (500mL×3); the organic phases were combined, and the organic phase was washed with saturated aqueous sodium chloride solution (500mL×2), dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain 56.1g of an oily product . Yield: 95%.
[0041] Preparation of intermediate II: under anhydrous and oxygen-free conditions, put 492mL lithium hexamethyldisilazide (2M) solution in 600mL tetrahydrofuran at -78°C, stir, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com