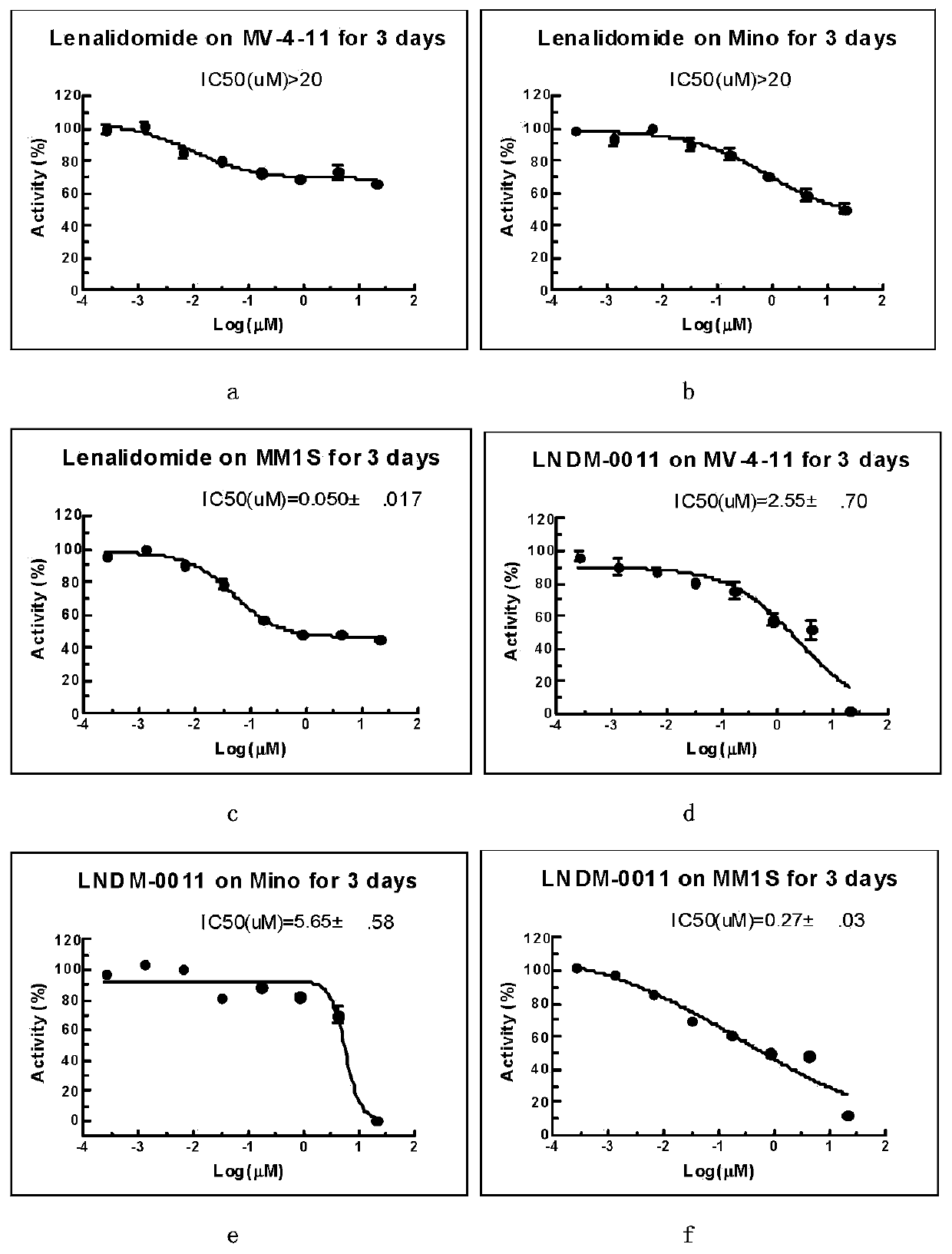
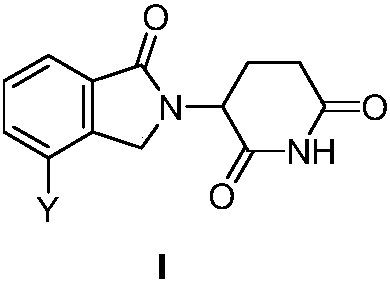
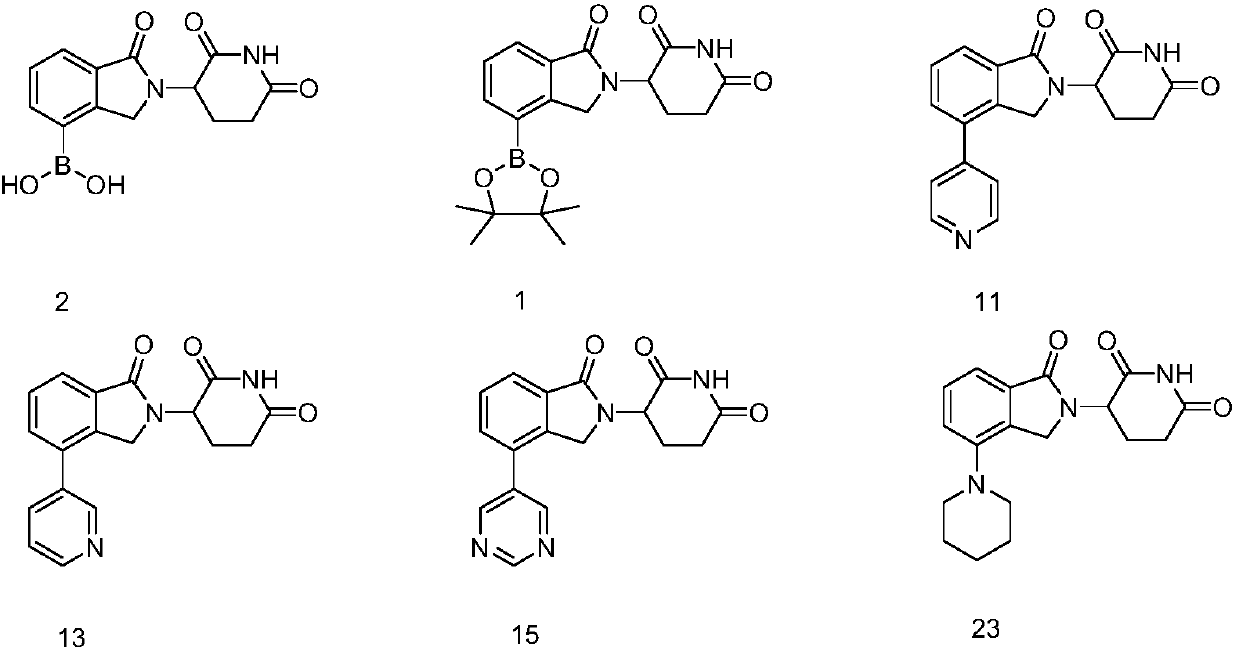
3-site substituted (1-iso-indoxoline-2-base)piperidine-2,6-thiazolidinedione and synthetic method thereof
A technology of oxoisoindoline and synthesis method, which is applied in the field of compound and synthesis, and can solve the problems of difficulty in synthesizing new compounds and many reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] 1.1 Synthesis of Compound 1
[0118]
[0119] Compound a (5.0g, 19.3mmol) and biboronic acid pinacol ester (5.4g, 21.26mmol) were added to a 250ml flask, 100ml of acetonitrile was added and stirred, then tert-butyl nitrite (3.44ml, 28.95mmol) was added , The reaction system was reacted at room temperature for 4 hours. The completion of the reaction was monitored by TLC, and the solvent was concentrated under reduced pressure to obtain a crude product. The crude product was separated and purified by silica gel column chromatography (dichloromethane:methanol=100:1) to obtain compound 1 as a white solid (3.86g, yield 54.1%) ).
[0120] 1 H-NMR (400MHz, DMSO) δ11.01 (s, 1H), 7.91 (d, J = 7.3Hz, 1H), 7.86 (d, J = 7.5Hz, 1H), 7.55 (t, J = 7.4Hz, 1H), 5.14(dd, J=13.3, 5.0Hz, 1H), 4.51(d, J=18.1Hz, 1H), 4.42(d, J= 18.1Hz, 1H), 2.98-2.82(m, 1H), 2.60 (d, J = 16.7Hz, 1H), 2.48-2.38 (m, 1H), 2.09-1.95 (m, 1H), 1.32 (s, 12H).
Embodiment 2
[0122] 1.2 Synthesis of Compound 2
[0123]
[0124] Compound 1 (1g, 2.7mmol) was added to a 100ml flask filled with tetrahydrofuran (20ml) and water (5ml), fully stirred, then slowly added sodium periodate (1.55g, 8.12mmol), and the system was stirred at room temperature for 2 Hour. 1M hydrochloric acid solution (1.89ml, 1.89mmol) was added to the reaction system, and stirred at room temperature for 12 hours. The organic solvent was removed from the reaction system under reduced pressure, then 30 ml of dichloromethane and 10 ml of water were added thereto, and the reaction system was fully stirred for one hour at room temperature. Compound 2 was collected by filtration and washed with water as a solid (523 mg, yield 67.3%).
[0125] 1 H NMR (400MHz, DMSO) δ10.98(s, 1H), 8.02(t, J=7.0Hz, 1H), 7.76(d, J=6.9Hz, 1H), 7.50 (t, J=7.5Hz, 1H ),5.13(dd,J=13.3,5.0Hz,1H),4.50(dd,J=36.4,18.1Hz,2H),3.17(s,2H),2.96–2.82(m,1H),2.59(d, J=17.1Hz, 1H), 2.45–2.36(m, 1H), 2.08–1.95(m, 1H...
Embodiment 3
[0127] 1.3 Synthesis of compound 9
[0128]
[0129] Compound 1 (200mg, 0.43mmol), compound 8 (115mg, 0.43mmol), potassium carbonate (176mg) was added in the flask of 100ml, the mixed solvent of the ethylene glycol dimethyl ether of 8ml and 1ml water was added thereto, then Add tetrakistriphenylphosphine palladium catalyst (25 mg), stir well, replace the reaction system with nitrogen several times, heat to 85 degrees, and react for 8 hours. Water was added to the reaction system, extracted with ethyl acetate, the organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. Filter and spin the filtrate to obtain a crude product. The crude product was separated and purified by silica gel column chromatography (dichloromethane:methanol=80:1) to obtain compound 9 as a solid (100mg, yield 45%)
[0130] 1 H NMR(400MHz,DMSO)δ10.98(s,1H),7.59(d,J=6.5Hz,1H),7.47(d,J=6.8Hz,2H),7.21 (q,J=8.0Hz,4H ),5.11(dd,J=13.2,5.0Hz,1H),4.39(d,J=17.2Hz,1H),4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com