Preparation method of cis-rosuvastatin calcium impurity
A technology of rosuvastatin calcium and cis, which is applied in the field of drug synthesis, can solve the problems such as difficulty in obtaining cis products, and achieve the effects of convenient post-processing, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
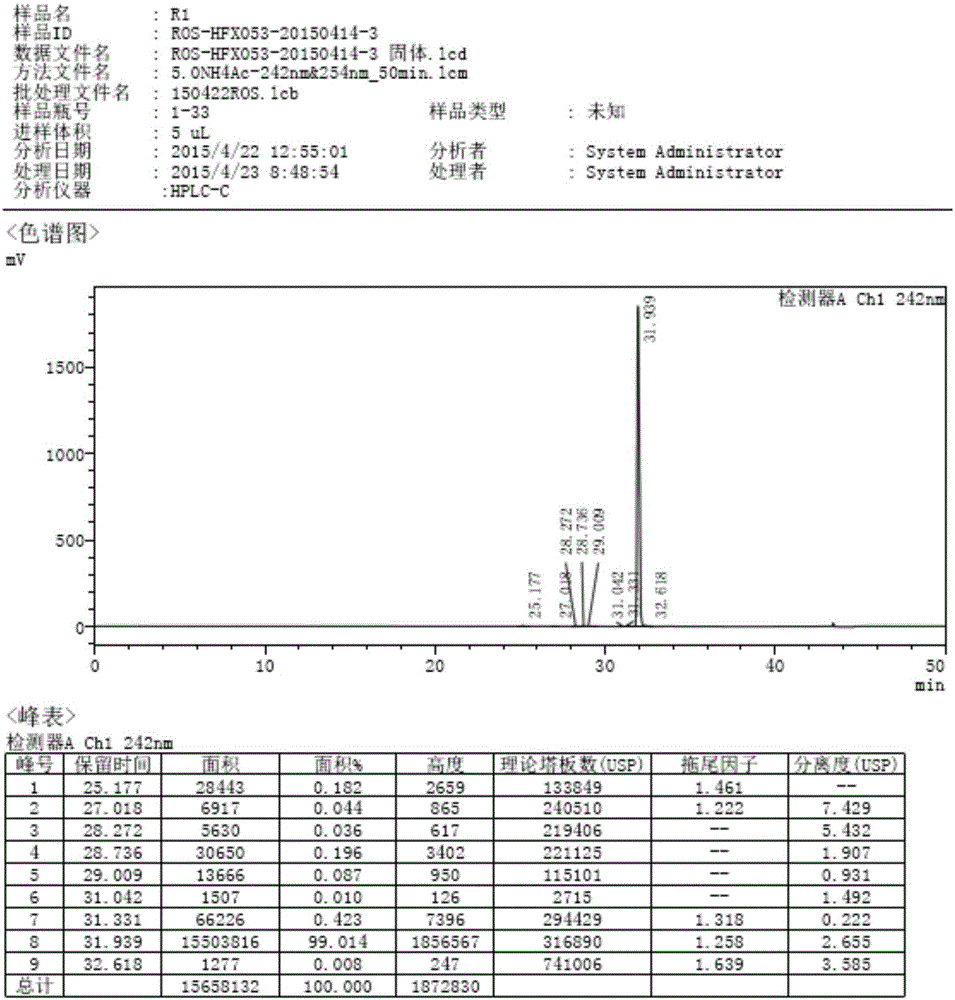
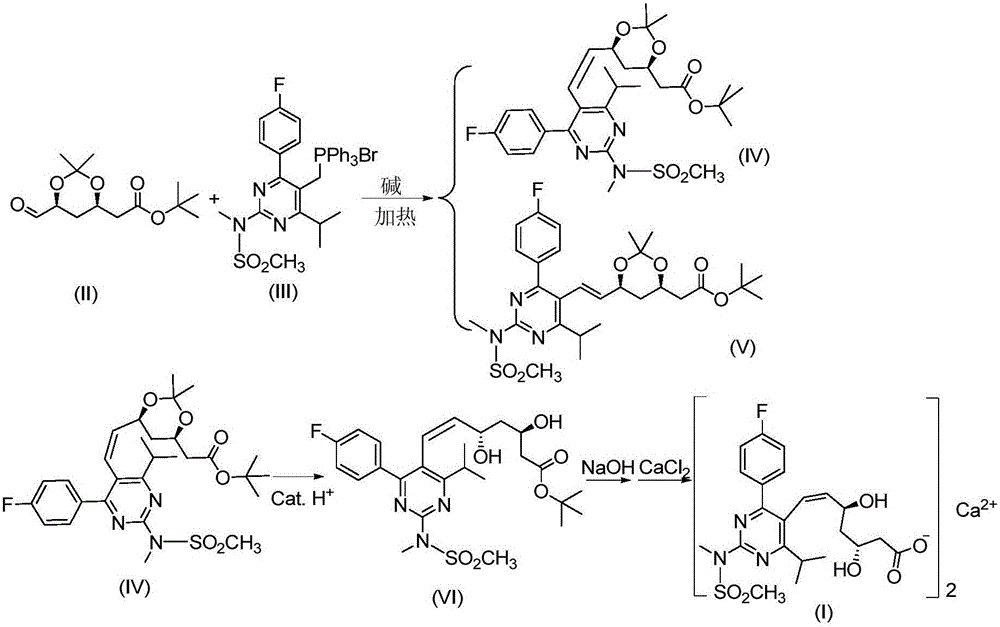
[0033] The compound of the present invention bis-[Z-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]-pyrimidin-5-yl](3R, The preparation method of 5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt (2:1) adopts the following steps:
[0034] (1) Compound (IV) 6-[(1Z)-2-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]-5-pyrimidine] Vinyl]-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate and compound (V) 6-[(1E)-2-[4-(4-fluorophenyl) )-6-isopropyl-2-[methyl(methylsulfonyl)amino]-5-pyrimidine]vinyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert Preparation of butyl ester:
[0035] Add DMSO 250ml and 19g D7((4R-cis)-6-carbaldehyde-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate) into a 1000mL four-necked flask, add Z8.2([4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonamido)-5-pyrimidinyl]triphenylphosphonium bromide) 45g , Add 20.8g of potassium carbonate, increase the temperature, keep at 75°C for 2h, after the Z8.2 reaction is complete, lower the ...
Embodiment 2
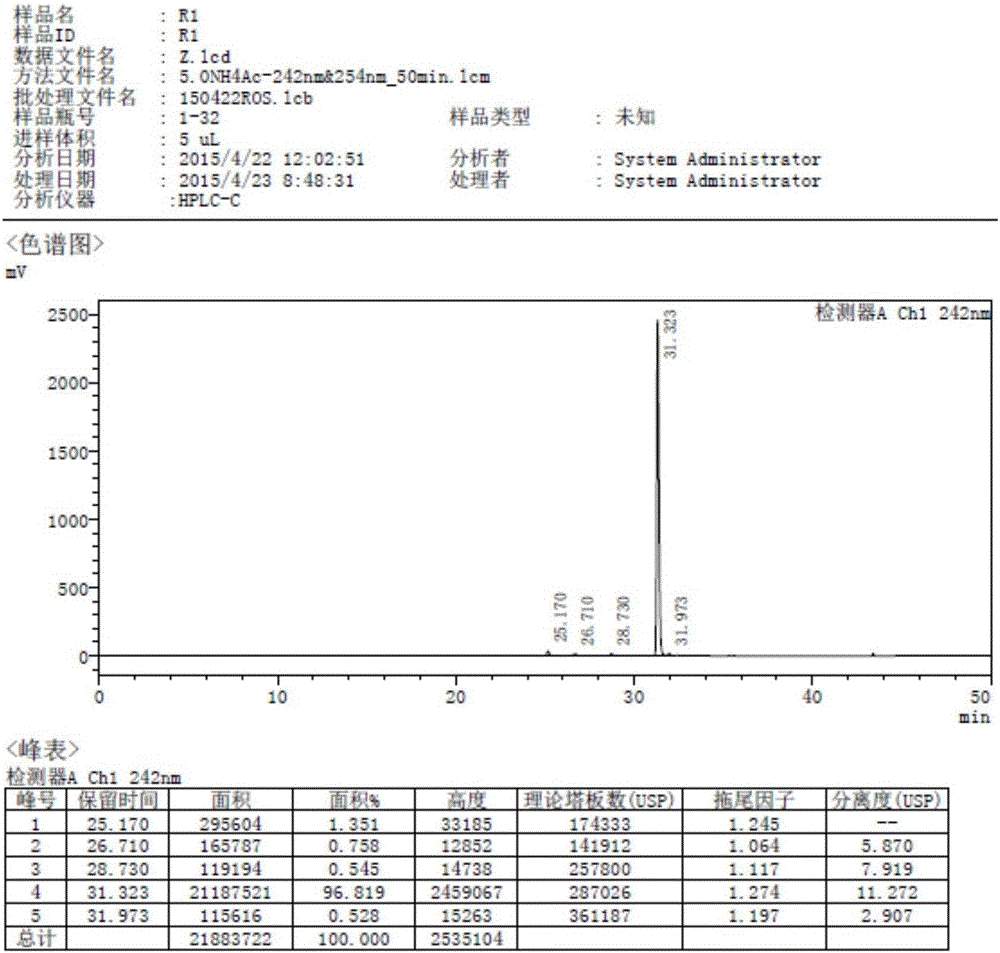
[0041] The compound of the present invention bis-[Z-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]-pyrimidin-5-yl](3R, The preparation method of 5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt (2:1) adopts the following steps:
[0042] (1) Compound (IV) 6-[(1Z)-2-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]-5-pyrimidine] Vinyl]-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate and compound (V) 6-[(1E)-2-[4-(4-fluorophenyl) )-6-isopropyl-2-[methyl(methylsulfonyl)amino]-5-pyrimidine]vinyl]-2,2-dimethyl-1,3-dioxane-4-acetic acid tert Preparation of butyl ester:
[0043] Add 250ml of DMF and 20g of D7 to a 1000mL four-necked flask, add Z8.245g, add 20.8g potassium carbonate, increase the temperature, keep it at 80°C for 2h, after the Z8.2 reaction is complete, reduce the temperature to 20°C, add 200ml of toluene, stir and filter. Add 200 ml of water to the organic layer and stir for 10 min. Separate the liquids, extract the aqueous layer with 100m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com