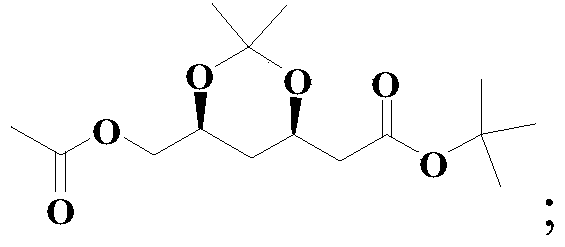
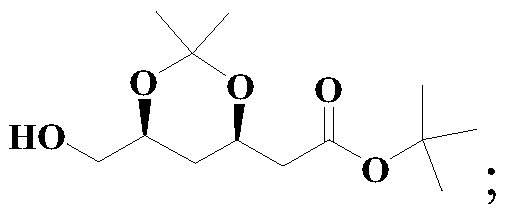
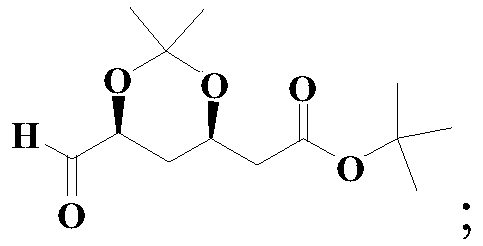
Method for preparing solid (4R-cis)-6-formyl-2,2-dimethyl-1,3-dioxane-4-tert-butyl acetate
A technology of tert-butyl acetate and 4r-cis, applied in the field of medicine and chemical industry, can solve problems such as difficult separation, storage, purification and purification, etc., and achieve the effects of simple operation, saving man-hours, and reducing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of the solid (4R-cis)-6-formyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester of this embodiment comprises the following steps:
[0050] (1) Dissolve 60.0g (0.198mol) of compound III in a mixed solvent of 360.0g methanol and 36.0g water, add 5.5g (0.0396mol) potassium carbonate, stir at 0°C for hydrolysis reaction, follow the reaction by GC, and complete the reaction (Compound III is less than <0.1%), remove potassium carbonate by filtration, rinse the filter cake with 20g of methanol, spin evaporate (45°C,-0.09Mpa) to remove the methanol in the filtrate, then add 480g of toluene to dissolve, wash with 200g×3 water, The toluene solution of compound II was obtained as a light yellow clear solution with a GC purity of 99.3%, which was directly used in the next oxidation reaction;
[0051] The structural formula of compound III is as follows:
[0052]
[0053] The structural formula of compound II is as follows:
[0054]
[0055] (2)...
Embodiment 2
[0060] The preparation method of the solid (4R-cis)-6-formyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester of this embodiment comprises the following steps:
[0061] (1) 60.0g (0.198mol) compound III is dissolved in the mixed solvent of 480.0g methanol and 24.0g, adds 13.7g (0.0991mol) potassium carbonate, stirs at 15 ℃ and carries out hydrolysis reaction, GC tracking reaction, reaction complete ( Compound III is less than < 0.1%), remove potassium carbonate by filtration, rinse the filter cake with 20g methanol, spin evaporate (45°C, -0.09Mpa) to remove methanol in the filtrate, then add 600g dichloromethane to dissolve, wash with 200g×3 , the dichloromethane solution of compound II was obtained, which was a light yellow clear solution with a GC purity of 99.5%, which was directly used in the next oxidation reaction;
[0062] The structural formula of compound III is as follows:
[0063]
[0064] The structural formula of compound II is as follows:
[0065] ...
Embodiment 3
[0071] The preparation method of the solid (4R-cis)-6-formyl-2,2-dimethyl-1,3-dioxane-4-acetic acid tert-butyl ester of this embodiment comprises the following steps:
[0072] (1) Dissolve 60.0g (0.198mol) of compound III Compound III in a mixed solvent dissolved in 480.0g of methanol and 4.8g, add 27.4g (0.198mol) of potassium carbonate, stir at 30°C for hydrolysis reaction, and follow the reaction by GC , after the reaction is complete (compound III is less than <0.1%), remove salt of wormwood by filtration, rinse the filter cake with 20g methanol, evaporate the methanol of the gained filtrate, then add 600g dichloromethane to dissolve, after 200g × 3 washings, obtain compound II Dichloromethane solution, GC purity 99.4%, directly used in the next oxidation reaction;
[0073] The structural formula of compound III is as follows:
[0074]
[0075] The structural formula of compound II is as follows:
[0076]
[0077] (2) Add 0.15g (0.99mmol) TEMPO, 2.36g (0.0198mol) p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com